DURATOCIN Solution for injection Ref.[27961] Active ingredients: Carbetocin
Source: Health Products and Food Branch (CA) Revision Year: 2018
Contraindications
Because of its long duration of action relative to oxytocin, uterine contractions produced by carbetocin cannot be stopped by simply discontinuing the medication. Therefore carbetocin should not be administered:
- Prior to delivery of the infant for any reason, including elective or medical induction of labour. Inappropriate use of carbetocin during pregnancy could theoretically mimic the symptoms of oxytocin over dosage, including hyperstimulation of the uterus with strong (hypertonic) or prolonged (tetanic) contractions, tumultuous labour, uterine rupture, cervical and vaginal lacerations, postpartum hemorrhage, utero-placental hypoperfusion and variable deceleration of fetal heart, fetal hypoxia, hypercapnia, or death.
- In patients with a history of hypersensitivity to oxytocin or carbetocin.
- In patients with serious cardiovascular disorders.
- Carbetocin is not intended for use in children.
Warnings and precautions
General
Duratocin should only be used at well-equipped specialist obstetrics units.
Some patients may not have an adequate uterine contraction after a single injection of DURATOCIN (carbetocin injection). In these patients, administration of DURATOCIN should not be repeated and more aggressive treatment with additional doses of other available uterotonic drugs like oxytocin or ergometrine is warranted.
In cases of persistent bleeding, the presence of retained placental fragments, coagulopathy, or trauma to the genital tract should be ruled out.
Carbetocin has antidiuretic effects. The risk of water intoxication cannot be excluded.
Patients with eclampsia and pre-eclampsia should be monitored for changes in blood pressure.
The safety of carbetocin in these patients has not been evaluated in formal clinical trials.
Cardiovascular
Should be used with extreme caution in patients with cardiovascular disease, especially coronary artery disease.
Endocrine and Metabolism
Specific studies have not been undertaken in gestational diabetes mellitus.
Neurologic
Should be used cautiously in the presence of migraine and epilepsy.
Respiratory
Should be used cautiously in the presence of asthma.
Special Populations
Pregnant Women
DURATOCIN (carbetocin injection) use during pregnancy, prior to the delivery of the infant, is contraindicated (see CONTRAINDICATIONS).
Nursing Women
Small amounts of carbetocin have been shown to cross over from plasma into the breast milk of nursing women who were given a 70 mcg dose intramuscularly, between 7 and 14 weeks postpartum. The mean peak concentration in breast milk was approximately 50 times lower than in plasma, and the ratio of the milk to plasma area under the concentration versus time curves (M/PAUC) was only 2-3%. The small amount of carbetocin transferred into breast milk or colostrum after a single injection, and subsequently ingested by a breast feeding infant, would not be expected to present a significant safety concern. This is due to the fact that carbetocin would be rapidly degraded by peptidases in the infant gastrointestinal tract.
Oxytocin is known to cause contraction of the myoepithelial cells surrounding the mammary alveoli, thereby stimulating milk let-down. There is no sufficient evidence to determine whether carbetocin can also stimulate milk let-down. However, milk let-down was found to occur normally in 5 nursing women after receiving a 70 mcg carbetocin dose by the intramuscular route.
Pediatrics (<18 years of age)
Not recommended for use.
Geriatrics (>65 years of age)
Not recommended for use.
Adverse reactions
Clinical Trial Adverse Drug Reactions
Because clinical trials are conducted under very specific conditions the adverse reaction rates observed in the clinical trials may not reflect the rates observed in practice and should not be compared to the rates in the clinical trials of another drug. Adverse drug reaction information from clinical trials is useful for identifying drug-related adverse events and for approximating rates.
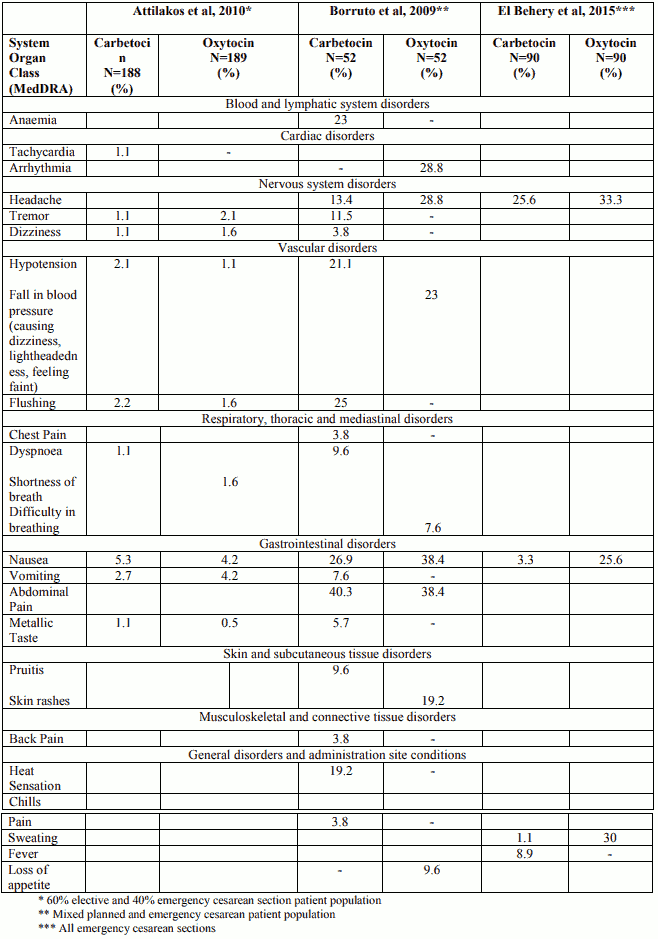
The more commonly observed adverse reactions in the clinical trials of patients undergoing elective cesarean section are summarized by frequency in Table 1 (Boucher, M. 199811, Dansereau, J. 199922, Barton, Scott R. et al, 19936)
Table 1. Very Common (≥10%) and Common (≥1% and <10%) Adverse Drug Reactions for Carbetocin in clinical trials of Elective Cesarean Section:
| System Organ Class | Very common ≥1/10 | Common ≥1/100 and <1/10 |
|---|---|---|
| Blood and lymphatic system disorders | Anaemia | |
| Nervous system disorders | Headache, tremor | Dizziness, anxiety |
| Vascular disorders | Hypotension, flushing | Tachycardia |
| Respiratory, thoracic and mediastinal disorders | Chest pain, dyspnoea | |
| Gastrointestinal disorders | Nausea, abdominal pain, vomiting | Metallic taste |
| Skin and subcutaneous tissue disorders | Pruritus | |
| Musculosceletal and connective tissue disorders | Back pain | |
| General disorders and administration site conditions | Feeling of warmth | Chills, pain, sweating |
The adverse drug reactions observed with carbetocin during the clinical trials were of the same type and frequency as the adverse events observed with oxytocin and placebo when administered after cesarean section under epidural or spinal anesthesia. The more commonly observed adverse reactions in the clinical trials of patients undergoing cesarean section are summarized by frequency in Table 2 (ref. Attilakos et al. 20105 , Borruto et al. 200910 and El Behery et al. 201525)
Table 2. Adverse Drug Reactions for Carbetocin (≥1%) in clinical trials of Cesarean Section:
The nature and frequency of the adverse drug reactions experienced by study participants receiving intravenous carbetocin were similar for patients undergoing either elective or emergency cesarean sections. Intravenous carbetocin was very commonly associated with anaemia, nausea, abdominal pain, pruritis, flushing, vomiting, feeling of warmth, hypotension, headache and tremor. Commonly associated adverse events included back pain, dizziness, metallic taste, sweating, chest pain, dyspnoea, chills, tachycardia and anxiety.
Drug interactions
During clinical trials, carbetocin has been administered in association with a number of analgesics, spasmolytics and agents used for epidural or spinal anaesthesia, and no drug interactions have been identified. However, dedicated interaction studies have not been undertaken.
No specific drug interactions have been reported with carbetocin, however since carbetocin is closely related in structure to oxytocin, it is possible that some of the same drug interactions known to be associated with oxytocin cannot be excluded.
Severe hypertension has been reported when oxytocin was given 3-4 hours following prophylactic administration of a vasoconstrictor in conjunction with caudal block anaesthesia.
Some inhalation-anaesthetics, such as cyclopropane may modify oxytocin’s cardiovascular effects, so as to produce unexpected results such as hypotension. Maternal sinus bradycardia with abnormal atrioventricular rhythms has also been noted when oxytocin was used concomitantly with cyclopropane anaesthesia.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.