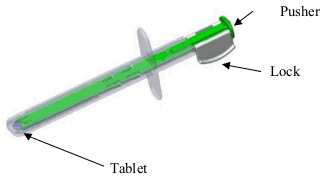
DZUVEO Sublingual tablet Ref.[49919] Active ingredients: Sufentanil
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Laboratoire Aguettant, 1, rue Alexander Fleming, 69007 Lyon, France
4.1. Therapeutic indications
Dzuveo is indicated for the management of acute moderate to severe pain in adult patients.
4.2. Posology and method of administration
Dzuveo is to be administered by a healthcare professional in a medically monitored setting only. A medically monitored setting must have equipment and personnel trained to detect and manage hypoventilation, and availability of supplemental oxygen and opioid antagonists, such as naloxone. Dzuveo should only be prescribed and administered by healthcare professionals who are experienced in the management of opioid therapy; particularly opioid adverse reactions, such as respiratory depression (see section 4.4).
Posology
Dzuveo is provided in a disposable single-dose applicator, to be administered by a healthcare provider as needed by the individual patient, but no more than once every hour, resulting in a maximum dose of 720 micrograms/day. Patients with a higher pain intensity at one hour after sufentanil treatment was initiated required more frequent redosing compared to patients with lower pain intensity scores at one hour.
Dzuveo should not be used beyond 48 hours.
Elderly
No specific dose adjustment is required in elderly patients. However, elderly patients should be observed closely for adverse reactions of sufentanil (see section 5.2).
Hepatic or renal impairment
Sufentanil should be administered with caution to patients with moderate to severe hepatic or severe renal impairment (see section 4.4).
Paediatric population
The safety and efficacy of sufentanil in children and adolescents below 18 years have not been established. No data are available.
Method of administration
For sublingual use only.
Dzuveo is to be administered by a healthcare professional from a disposable single-dose applicator (see section 6.6). The applicator is used as a placement aid for the healthcare professional to deliver the tablet under the tongue, on an as needed basis, per patient request, with a minimum of 1 hour between doses.
The dispensed sublingual tablet should dissolve under the tongue and should not be chewed or swallowed. If swallowed, the oral bioavailability of Dzuveo is only 9% which would result in a sub-therapeutic dose. Patients should not eat or drink and should minimise talking for 10 minutes after each dose of sufentanil 30 mcg sublingual tablet. In the case of an excessive dry mouth, patients may be given ice cubes. Some insoluble excipients of the tablet may remain in the mouth after dissolution is complete; this is normal and does not indicate lack of absorption of sufentanil from the tablet.
See section 6.6 for instructions regarding handling of the Dzuveo sublingual tablet and applicator.
4.9. Overdose
Signs and symptoms
Sufentanil overdose is manifested by an exaggeration of its pharmacological effects. Depending on individual sensitivity, the clinical picture is determined by the degree of respiratory depression. This may range from hypoventilation to respiratory arrest. Other symptoms that may occur are loss of consciousness, coma, cardiovascular shock and muscle rigidity.
Management
Management of sufentanil overdose should be focused on treating symptoms of μ-opioid receptor agonism, including administration of oxygen. Primary attention should be given to obstruction of airways and the necessity of assisted or controlled ventilation.
An opiate antagonist (e.g. naloxone) should be administered in the event of respiratory depression. This does not rule out more direct countermeasures. The shorter duration of activity of the opiate antagonist compared to that of sufentanil should be taken into account. In that case, the opioid antagonist can be administered repeatedly or by infusion.
6.3. Shelf life
4 years.
6.4. Special precautions for storage
Store in the original package in order to protect from light and oxygen.
6.5. Nature and contents of container
Dzuveo is packaged in a polypropylene single-dose applicator, which is packaged in a polyester film/LDPE/aluminium foil/LDPE sachet with an oxygen absorber.
Dzuveo will be available in cartons of 5 and 10. Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Instructions for use of Single Dose Applicator (SDA)
Single-Use Product / Do Not Reuse
Do Not Use if Pouch Seal is Broken
Do not use if the Single Dose Applicator (SDA) is damaged
Instruct the patient to not chew or swallow the tablet.
Instruct the patient to not eat or drink and minimize talking for 10 minutes after receiving the tablet.
1. When ready to administer the medicine, tear open the slit-notched pouch across the top. The pouch contains one clear plastic SDA with a single blue-colored tablet housed in the tip, and an oxygen absorber packet. The oxygen absorber packet should be discarded.
Contents of the pouch are shown below:
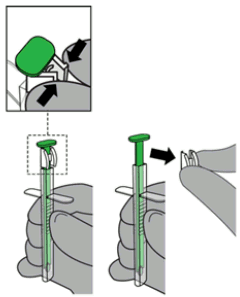
2. Remove the white Lock from the green Pusher by squeezing the sides together and detaching from Pusher. Discard the Lock.
3. Tell the patient to touch their tongue to the roof of their mouth if possible.
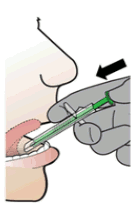
4. Rest the SDA lightly on the patient’s teeth or lips.
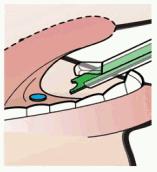
5. Place the SDA tip under the tongue and aim at the floor of the patient’s mouth.
NOTE: Avoid direct mucosal contact with the SDA tip.
6. Depress the green Pusher to deliver the tablet to the patient’s sublingual space and confirm tablet placement.
The single-dose applicator (SDA) must be discarded in accordance to the institutional policies and local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.