EBANGA Solution for injection Ref.[50362] Active ingredients: Ansuvimab
Source: FDA, National Drug Code (US) Revision Year: 2022
12.1. Mechanism of Action
Ansuvimab-zykl is a recombinant human monoclonal antibody with antiviral activity against Zaire ebolavirus [see Microbiology (12.4)].
12.2. Pharmacodynamics
Ansuvimab-zykl exposure-response relationship and the time course of pharmacodynamic response is unknown.
12.3. Pharmacokinetics
Limited data from 18 healthy subjects 22 to 56 years of age suggests that the pharmacokinetic profile of ansuvimab-zykl is consistent with the profile of other IgG1 monoclonal antibodies.
Pharmacokinetic data are not available for Zaire ebolavirus infected patients.
Specific Populations
The effect of age, renal impairment or hepatic impairment on the pharmacokinetics of ansuvimab-zykl is unknown.
12.4. Microbiology
Mechanism of Action
EBANGA (ansuvimab-zykl) is a recombinant, human IgG1κ monoclonal antibody that binds to the glycan cap and inner chalice of the EBOV GP1 subunit. The epitope to which it binds is located within the receptor binding domain of EBOV consisting of amino acids LEIKKPDGS (GP residues 111–119).
Ansuvimab-zykl binds EBOV GP without the mucin domain with a KD of 0.2 nM at pH 7.4 and 0.6 nM at pH 5.3 as measured by biolayer interferometry. Ansuvimab-zykl blocks binding of EBOV GP1 to the Neiman Pick cell receptor 1 in host cells (IC50 value of 0.09 μg/mL), inhibiting virus entry into the host cell. Ansuvimab-zykl exhibited Fc-mediated Antibody Dependent Cellular Cytotoxicity (ADCC) activity against cells expressing EBOV GP when effector cells were added.
Antiviral Activity
In a live virus plaque-reduction neutralization assay performed in Vero E6 cells, ansuvimab-zykl neutralized Zaire ebolavirus Mayinga with an EC50 value of 0.06 µg/mL. In an EBOV GP lentivirus infectivity assay using HEK293 cells, ansuvimab-zykl inhibited Zaire ebolavirus Mayinga with an EC50 value of 0.09 μg/mL and Zaire ebolavirus Makona with an EC50 value of 0.15 μg/mL. The ADCC activity of ansuvimab-zykl was assessed in EBOV GP-transduced and non-transduced HEK293T target cells in the presence of antibody with effector cells added at an effector-to-target cell ratio 1:50 and analyzed via flow cytometry. Ansuvimab-zykl mediated ADCC, with maximal activity observed at a mAb concentration of 0.03 μg/mL. Treatment of Zaire ebolavirus infected rhesus macaques with a single IV dose of ansuvimab-zykl (50 mg per kg) generally protected infected animals from Zaire ebolavirus mediated death when drug was administered 5 days post-infection.
Resistance
No nonclinical or clinical studies evaluating resistance to ansuvimab-zykl have been conducted. The possibility of resistance to ansuvimab-zykl should be considered in patients who either fail to respond to therapy or who develop relapse of disease after an initial period of responsiveness.
Immune Response
Interaction studies with recombinant live EBOV vaccines and EBANGA have not been conducted [see Drug Interactions (7.1)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, genotoxicity and fertility studies have not been conducted with ansuvimab-zykl.
14. Clinical Studies
The efficacy of EBANGA has been evaluated in 174 subjects with confirmed Zaire ebolavirus infection in the PALM trial, a multi-center, open-label, randomized, controlled trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID; NCT03719586). The trial was conducted in the North Kivu and Ituri provinces in the Democratic Republic of Congo, where an outbreak began in August 2018, and enrolled 681 subjects of all ages, including pregnant women, with documented Zaire ebolavirus infection and symptoms of any duration who were receiving oSOC. Subjects were randomized to receive EBANGA 50 mg/kg IV as a single infusion, an investigational control 50 mg/kg IV every third day, for a total of 3 doses, or other investigational drugs. Eligible subjects had a positive reverse transcriptase-polymerase chain reaction (RT-PCR) for the nucleoprotein (NP) gene of Zaire ebolavirus and had not received other investigational treatments (with the exception of experimental vaccines) within the previous 30 days. Neonates ≤7 days of age were eligible if the mother had documented infection. Neonates born to a mother who had cleared Zaire ebolavirus following a course of her assigned investigational medication were also eligible to be enrolled at investigator discretion regarding the likelihood that the neonate was infected. Randomization was stratified by reverse transcription-PCR cycle threshold calculated using NP targets (CtNP ≤22.0 vs >22.0; corresponding to high and low viral load, respectively) and Ebola Treatment Unit (ETU) site. All subjects received oSOC consisting, at a minimum, of IV fluids, daily clinical laboratory testing, correction of hypoglycemia and electrolyte imbalances, and broad-spectrum antibiotics and antimalarials, as indicated.
The primary efficacy endpoint was 28-day mortality. The primary analysis population includes all subjects who were randomized and concurrently eligible to receive either EBANGA or the investigational control during the same time period of the trial.
The demographics and baseline characteristics are provided in Table 4 below.
Table 4. Demographics and Baseline Characteristics in PALM Trial:
| Parameter | EBANGA N=174 N (%) | Control N=168 N (%) |
|---|---|---|
| Mean age (years) | 27.3 | 29.9 |
| Age <1 month, n (%) | 4 (2) | 2 (1) |
| Age 1 month to <1 year, n (%) | 7 (4) | 5 (3) |
| Age 1 year to <6 years, n (%) | 15 (9) | 12 (7) |
| Age 6 years to <12 years, n (%) | 13 (7) | 5 (3) |
| Age 12 years to <18 years, n (%) | 15 (9) | 9 (5) |
| Age 18 years to <50 years, n (%) | 93 (53) | 114 (68) |
| Age 50 years to <65 years, n (%) | 21 (12) | 18 (11) |
| Age ≥65 years, n (%) | 6 (3) | 3 (2) |
| Female, n (%) | 98 (56) | 87 (52) |
| Positive result on pregnancy test*, n (%) | 5/98 (5) | 4/87 (5) |
| RT-PCR CtNP cycle threshold ≤22, n (%) | 73 (42) | 70 (42) |
| Median RT-PCR CtNP (IQR) | 23.3 (19.7, 28.5) | 23.1 (19.0, 26.5) |
| Median creatinine (IQR) | 0.9 (0.6, 2.4) | 1.2 (0.8, 4.3) |
| Median AST (IQR) | 234 (66, 978) | 351 (112, 1404) |
| Median ALT (IQR) | 168 (44, 551) | 236 (48, 631) |
| Median days from onset of symptoms to randomization (IQR) | 5 (3, 7) | 5 (3, 7) |
| Reported Vaccination with rVSV-ZEBOV vaccine, n (%) | 36 (21) | 41 (24) |
| <10 days before ETU admission, n (%) | 22/36 (61) | 21/41 (51) |
| ≥10 days before ETU admission, n (%) | 12/36 (33) | 18/41 (44) |
| Timing unknown, n (%) | 2/36 (6) | 2/41 (5) |
CtNP = cycle threshold calculated using NP targets; IQR=interquartile range; AST=Aspartate aminotransferase; ALT=Alanine aminotransferase; ETU=Ebola treatment unit.
* Pregnancy positive test was calculated based on subjects who were pregnant. Denominator for percentages is the number of females in the treatment group.
The PALM trial was stopped early on the basis of a pre-specified interim analysis showing a statistically significant reduction in mortality for EBANGA compared to control assessed at Day 28.
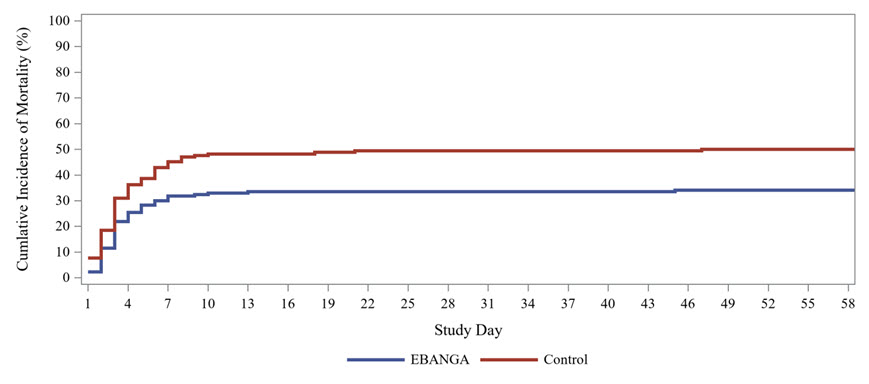
Mortality efficacy results are shown in Table 5 and Figure 1.
Table 5. Mortality Rates in PALM Trial:
| Efficacy Endpoints | EBANGA* N=174 | Control* N=168 |
|---|---|---|
| Overall | ||
| 28-day mortality, n (%) | 61 (35%) | 83 (49%) |
| Mortality rate difference relative to control (95% CI)† | -14.3 (-24.7, -3.7) | |
| p-Value‡ | 0.008 | |
| Baseline Viral Load | ||
| High viral load (CtNP ≤22)§ | ||
| 28-day mortality, n (%) | 51/73 (70%) | 60/70 (86%) |
| Mortality rate difference relative to control (95% CI)† | -15.9 (-31.6, 0.9) | |
| Low viral load (CtNP >22)§ | ||
| <28-day mortality, n (%) | 10/101 (10%) | 23/97 (24%) |
| Mortality rate difference relative to control (95% CI)† | -13.8 (-27.3, 0.3) | |
| Age group, 28-day mortality, n/N (%) | ||
| Adults (age ≥18 years) | 41/120 (34%) | 68/135 (50%) |
| <12 to <18 years of age | 5/15 (33%) | 5/9 (56%) |
| <6 to <12 years of age | 4/13 (31%) | 2/5 (40%) |
| <6 years of age | 11/26 (42%) | 8/19 (42%) |
| Sex, 28-day mortality, n/N (%) | ||
| Male | 30/76 (39%) | 32/81 (40%) |
| Female | 31/98 (32%) | 51/87 (59%) |
N=Number of subjects in the Concurrent Intention-to-Treat population and treatment group; n=Number of subjects with the 28-day outcome. Denominator for percentages is the total number of subjects in the specific group.
* Both EBANGA and Control were administered with optimized standard of care
† 95% CI for Difference = 95% confidence intervals were computed by inverting two one-sided exact tests.
‡ The result is significant according to the interim stopping boundary, p<0.028.
§ Cepheid GeneXpert Ebola Assay used for detection of Zaire ebolavirus RNA
Figure 1. Kaplan-Meier Curve for Overall Mortality in PALM Trial:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.