EDECRIN Tablet Ref.[10133] Active ingredients: Etacrynic acid
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
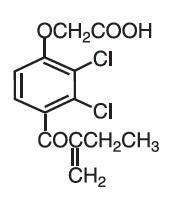
Ethacrynic acid is an unsaturated ketone derivative of an aryloxyacetic acid. It is designated chemically as [2,3-dichloro-4-(2-methylene-1-oxobutyl)phenoxy] acetic acid, and has a molecular weight of 303.14. Ethacrynic acid is a white, or practically white, crystalline powder, very slightly soluble in water, but soluble in most organic solvents such as alcohols, chloroform, and benzene. Its empirical formula is C13H12Cl2O4 and its structural formula is:
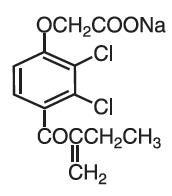
Ethacrynate sodium, the sodium salt of ethacrynic acid, is soluble in water at 25°C to the extent of about 7 percent. Solutions of the sodium salt are relatively stable at about pH 7 at room temperature for short periods, but as the pH or temperature increases the solutions are less stable. The molecular weight of ethacrynate sodium is 325.12. Its empirical formula is C13H11Cl2NaO4 and its structural formula is:
EDECRIN is supplied as 25 mg tablets for oral use. The tablets contain the following inactive ingredients: colloidal silicon dioxide, lactose, magnesium stearate, starch and talc. Intravenous SODIUM EDECRIN (ethacrynate sodium) is a sterile freeze-dried powder and is supplied in a vial containing:
Ethacrynate sodium equivalent to ethacrynic acid 50.0 mg.
Inactive ingredient: Mannitol 62.5 mg
| How Supplied |
|---|
|
Tablets EDECRIN, 25 mg, are white, capsule shaped, scored tablets, coded VRX 205 on one side and EDECRIN on the other. They are supplied as follows: NDC 25010-215-15 in bottles of 100. Intravenous SODIUM EDECRIN is a dry white material either in a plug form or as a powder. It is supplied in vials containing ethacrynate sodium equivalent to 50 mg of ethacrynic acid. NDC 25010-210-27 EDECRIN Tablets (ethacrynic acid): Distributed by: Bausch Health US, LLC, Bridgewater, NJ 08807 USA Manufactured by: Bausch Health Companies Inc., Steinbach, MB R5G 1Z7, Canada SODIUM EDECRIN Intravenous (ethacrynate sodium): Distributed by: Bausch Health US, LLC, Bridgewater, NJ 08807 USA Manufactured by: Patheon Manufacturing Services LLC, Greenville, NC 27834 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| EDECRIN | Australia, Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.