EFAVIRENZ / EMTRICITABINE / TENOFOVIR DISOPROXIL MYLAN Film-coated tablet Ref.[107686] Active ingredients: Emtricitabine, Tenofovir disoproxil and Efavirenz
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Mylan Pharmaceuticals Limited, Damastown Industrial Park, Mulhuddart, Dublin 15, DUBLIN, Ireland
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Severe hepatic impairment (CPT, Class C) (see section 5.2).
Co-administration with terfenadine, astemizole, cisapride, midazolam, triazolam, pimozide, bepridil, or ergot alkaloids (for example, ergotamine, dihydroergotamine, ergonovine, and methylergonovine). Competition for cytochrome P450 (CYP) 3A4 by efavirenz could result in inhibition of metabolism and create the potential for serious and/or life-threatening adverse reactions (for example, cardiac arrhythmias, prolonged sedation or respiratory depression) (see section 4.5).
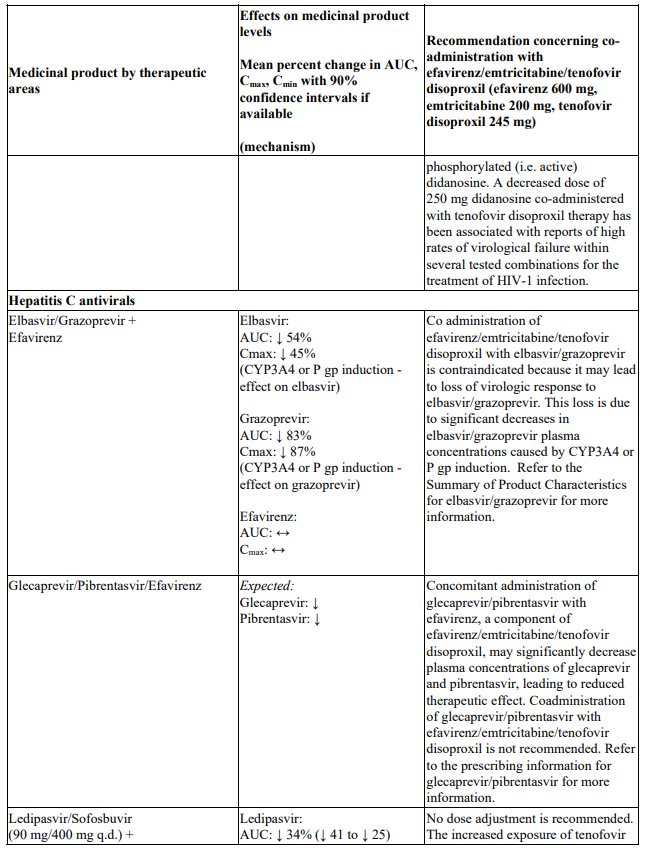
Co administration with elbasvir/grazoprevir due to the expected significant decreases in plasma concentrations of elbasvir and grazoprevir. This effect is due to induction of CYP3A4 or P-gp by efavirenz and may result in loss of therapeutic effect of elbasvir/grazoprevir (see section 4.5).
Co-administration with voriconazole. Efavirenz significantly decreases voriconazole plasma concentrations while voriconazole also significantly increases efavirenz plasma concentrations. Since Efavirenz/Emtricitabine/Tenofovir disoproxil Mylan is a fixed-dose combination product, the dose of efavirenz cannot be altered (see section 4.5).
Co-administration with herbal preparations containing St. John’s wort (Hypericum perforatum) due to the risk of decreased plasma concentrations and reduced clinical effects of efavirenz (see section 4.5).
Administration to patients with:
- a family history of sudden death or of congenital prolongation of the QTc interval on electrocardiograms, or with any other clinical condition known to prolong the QTc interval.
- a history of symptomatic cardiac arrhythmias or with clinically relevant bradycardia or with congestive cardiac failure accompanied by reduced left ventricle ejection fraction.
- severe disturbances of electrolyte balance e.g. hypokalemia or hypomagnesemia.
Co-administration with medicinal products that are known to prolong the QTc interval (proarrhythmic).
These medicinal products include:
- antiarrhythmics of classes IA and III,
- neuroleptics, antidepressive agents,
- certain antibiotics including some agents of the following classes: macrolides, fluoroquinolones, imidazole and triazole antifungal agents,
- certain non-sedating antihistamines (terfenadine, astemizole),
- cisapride,
- flecainide,
- certain antimalarials,
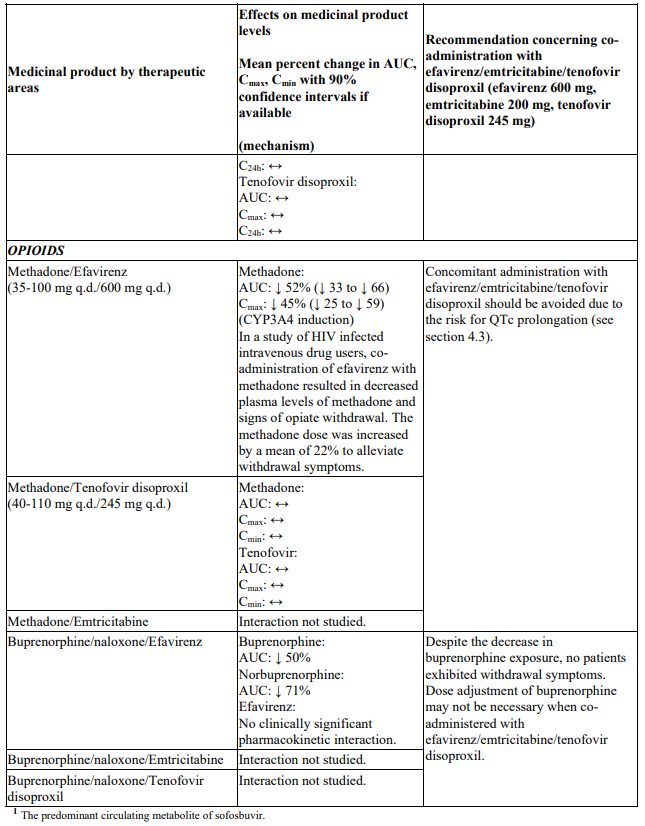
- methadone (see sections 4.4, 4.5 and 5.1).
4.4. Special warnings and precautions for use
Co-administration with other medicinal products
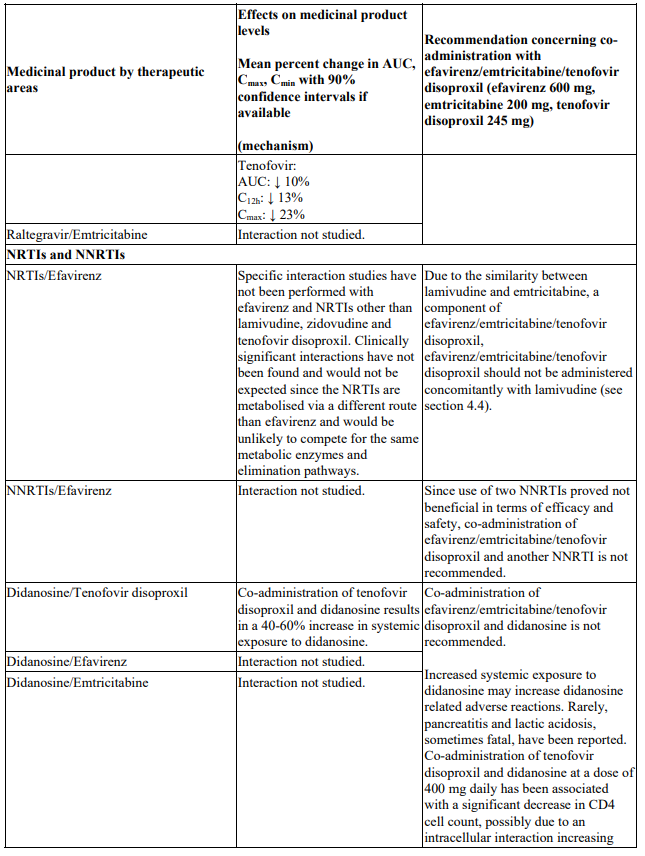
As a fixed combination, efavirenz/emtricitabine/tenofovir disoproxil should not be administered concomitantly with other medicinal products containing the same active components, emtricitabine or tenofovir disoproxil. Efavirenz/emtricitabine/tenofovir disoproxil should not be co-administered with products containing efavirenz unless needed for dose adjustment e.g. with rifampicin (see section 4.2). Due to similarities with emtricitabine, efavirenz/emtricitabine/tenofovir disoproxil should not be administered concomitantly with other cytidine analogues, such as lamivudine (see section 4.5). Efavirenz/emtricitabine/tenofovir disoproxil should not be administered concomitantly with adefovir dipivoxil or with medicinal products containing tenofovir alafenamide.
Co-administration of efavirenz/emtricitabine/tenofovir disoproxil and didanosine is not recommended (see section 4.5).
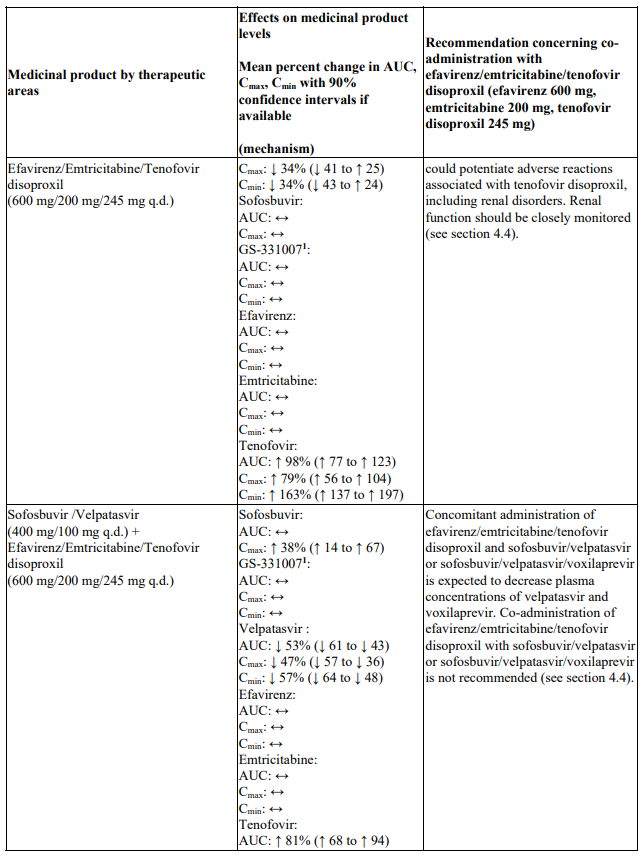
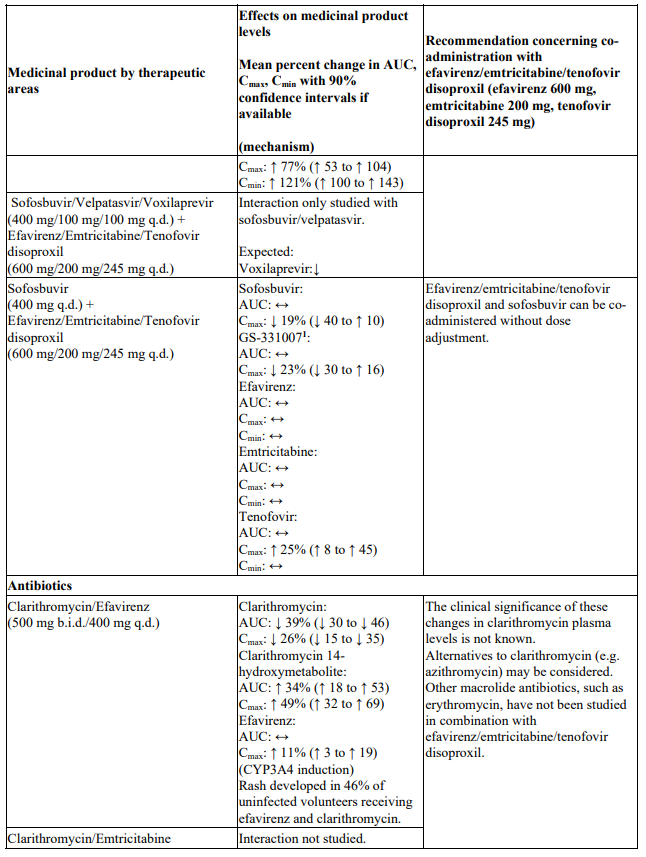
Co-administration of efavirenz/emtricitabine/tenofovir disoproxil and sofosbuvir/velpatasvir or sofosbuvir/velpatasvir/voxilaprevir is not recommended since plasma concentrations of velpatasvir and voxilaprevir are expected to decrease following co-administration with efavirenz leading to reduced therapeutic effect of sofosbuvir/velpatasvir or sofosbuvir/velpatasvir/voxilaprevir (see section 4.5).
No data are available on the safety and efficacy of efavirenz/emtricitabine/tenofovir disoproxil in combination with other antiretroviral agents.
Concomitant use of Ginkgo biloba extracts is not recommended (see section 4.5).
Switching from a Protease Inhibitor (PI)-based antiretroviral regimen
Currently available data indicate a trend that in patients on a PI-based antiretroviral regimen the switch to efavirenz/emtricitabine/tenofovir disoproxil may lead to a reduction of the response to the therapy (see section 5.1). These patients should be carefully monitored for rises in viral load and, since the safety profile of efavirenz differs from that of protease inhibitors, for adverse reactions.
Opportunistic infections
Patients receiving efavirenz/emtricitabine/tenofovir disoproxil or any other antiretroviral therapy may continue to develop opportunistic infections and other complications of HIV infection, and therefore should remain under close clinical observation by physicians experienced in the treatment of patients with HIV associated diseases.
Effect of food
The administration of efavirenz/emtricitabine/tenofovir disoproxil with food may increase efavirenz exposure (see section 5.2) and may lead to an increase in frequency of adverse reactions (see section 4.8). It is recommended that efavirenz/emtricitabine/tenofovir disoproxil be taken on an empty stomach, preferably at bedtime.
Liver disease
The pharmacokinetics, safety and efficacy of efavirenz/emtricitabine/tenofovir disoproxil have not been established in patients with significant underlying liver disorders (see section 5.2). Efavirenz/emtricitabine/tenofovir disoproxil is contraindicated in patients with severe hepatic impairment (see section 4.3) and not recommended in patients with moderate hepatic impairment. Since efavirenz is principally metabolised by the CYP system, caution should be exercised in administering efavirenz/emtricitabine/tenofovir disoproxil to patients with mild hepatic impairment. These patients should be carefully monitored for efavirenz adverse reactions, especially nervous system symptoms. Laboratory tests should be performed to evaluate their liver disease at periodic intervals (see section 4.2).
Patients with pre-existing liver dysfunction including chronic active hepatitis have an increased frequency of liver function abnormalities during combination antiretroviral therapy (CART) and should be monitored according to standard practice. If there is evidence of worsening liver disease or persistent elevations of serum transaminases to greater than 5 times the upper limit of the normal range, the benefit of continued therapy with efavirenz/emtricitabine/tenofovir disoproxil needs to be weighed against the potential risks of significant liver toxicity. In such patients, interruption or discontinuation of treatment must be considered (see section 4.8).
In patients treated with other medicinal products associated with liver toxicity, monitoring of liver enzymes is also recommended.
Hepatic events
Post-marketing reports of hepatic failure also occurred in patients with no pre-existing hepatic disease or other identifiable risk factors (see section 4.8). Liver enzyme monitoring should be considered for all patients independent of pre-existing hepatic dysfunction or other risk factors.
Patients with HIV and hepatitis B (HBV) or C virus (HCV) co-infection
Patients with chronic hepatitis B or C and treated with CART are at an increased risk for severe and potentially fatal hepatic adverse reactions.
Physicians should refer to current HIV treatment guidelines for the optimal management of HIV infection in patients co-infected with HBV.
In case of concomitant antiviral therapy for hepatitis B or C, please refer also to the relevant Summary of Product Characteristics for these medicinal products.
The safety and efficacy of efavirenz/emtricitabine/tenofovir disoproxil have not been studied for the treatment of chronic HBV infection. Emtricitabine and tenofovir individually and in combination have shown activity against HBV in pharmacodynamic studies (see section 5.1). Limited clinical experience suggests that emtricitabine and tenofovir disoproxil have an anti-HBV activity when used in antiretroviral combination therapy to control HIV infection. Discontinuation of efavirenz/emtricitabine/tenofovir disoproxil therapy in patients co-infected with HIV and HBV may be associated with severe acute exacerbations of hepatitis. Patients co-infected with HIV and HBV who discontinue efavirenz/emtricitabine/tenofovir disoproxil must be closely monitored with both clinical and laboratory follow-up for at least four months after stopping treatment with efavirenz/emtricitabine/tenofovir disoproxil. If appropriate, resumption of anti-hepatitis B therapy may be warranted. In patients with advanced liver disease or cirrhosis, treatment discontinuation is not recommended since post-treatment exacerbation of hepatitis may lead to hepatic decompensation.
QTc prolongation
QTc prolongation has been observed with the use of efavirenz (see sections 4.5 and 5.1). For patients at increased risk of Torsade de Pointes or who are receiving medicinal products with a known risk for Torsade de Pointes, consider alternatives to efavirenz/emtricitabine/tenofovir disoproxil.
Psychiatric symptoms
Psychiatric adverse reactions have been reported in patients treated with efavirenz. Patients with a prior history of psychiatric disorders appear to be at greater risk of serious psychiatric adverse reactions. In particular, severe depression was more common in those with a history of depression. There have also been post-marketing reports of severe depression, death by suicide, delusions, psychosis-like behaviour, and catatonia. Patients should be advised that if they experience symptoms such as severe depression, psychosis or suicidal ideation, they should contact their doctor immediately to assess the possibility that the symptoms may be related to the use of efavirenz, and if so, to determine whether the risk of continued therapy outweighs the benefits (see section 4.8).
Nervous system symptoms
Symptoms including, but not limited to, dizziness, insomnia, somnolence, impaired concentration and abnormal dreaming are frequently reported undesirable effects in patients receiving efavirenz 600 mg daily in clinical studies. Dizziness was also seen in clinical studies with emtricitabine and tenofovir disoproxil. Headache has been reported in clinical studies with emtricitabine (see section 4.8). Nervous system symptoms associated with efavirenz usually begin during the first one or two days of therapy and generally resolve after the first two to four weeks. Patients should be informed that if they do occur, these common symptoms are likely to improve with continued therapy and are not predictive of subsequent onset of any of the less frequent psychiatric symptoms.
Seizures
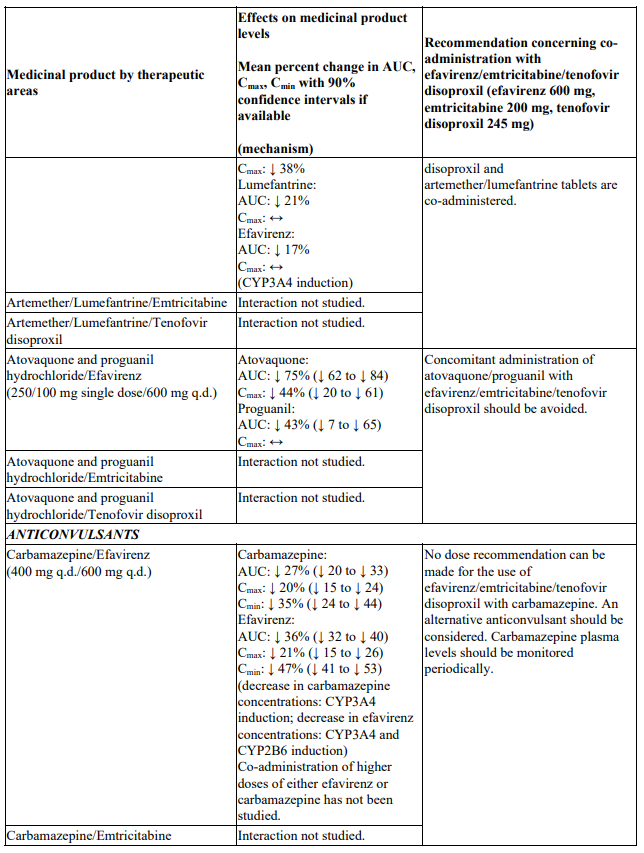
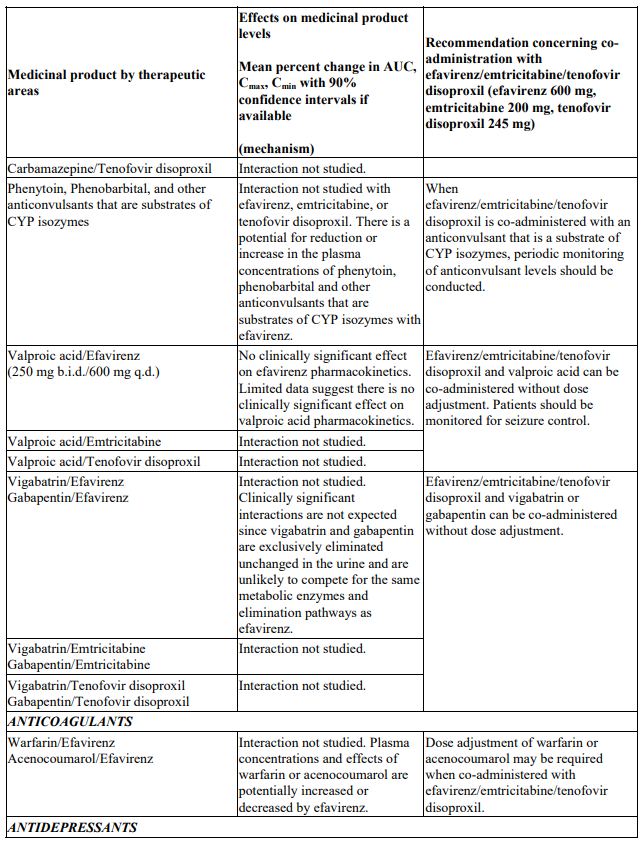
Convulsions have been observed in patients receiving efavirenz, generally in the presence of a known medical history of seizures. Patients who are receiving concomitant anticonvulsant medicinal products primarily metabolised by the liver, such as phenytoin, carbamazepine and phenobarbital, may require periodic monitoring of plasma levels. In a medicinal product interaction study, carbamazepine plasma concentrations were decreased when carbamazepine was co-administered with efavirenz (see section 4.5). Caution must be taken in any patient with a history of seizures.
Renal impairment
Efavirenz/emtricitabine/tenofovir disoproxil is not recommended for patients with moderate or severe renal impairment (creatinine clearance < 50 ml/min). Patients with moderate or severe renal impairment require a dose adjustment of emtricitabine and tenofovir disoproxil that cannot be achieved with the combination tablet (see sections 4.2 and 5.2). Use of Efavirenz/emtricitabine/tenofovir disoproxil should be avoided with concurrent or recent use of a nephrotoxic medicinal product. If concomitant use of efavirenz/emtricitabine/tenofovir disoproxil and nephrotoxic agents (e.g. aminoglycosides, amphotericin B, foscarnet, ganciclovir, pentamidine, vancomycin, cidofovir, interleukin-2) is unavoidable, renal function must be monitored weekly (see section 4.5).
Cases of acute renal failure after initiation of high dose or multiple non-steroidal anti-inflammatory drugs (NSAIDs) have been reported in patients treated with tenofovir disoproxil and with risk factors for renal dysfunction. If efavirenz/emtricitabine/tenofovir disoproxil is co-administered with an NSAID, renal function should be monitored adequately.
Renal failure, renal impairment, elevated creatinine, hypophosphataemia and proximal tubulopathy (including Fanconi syndrome) have been reported with the use of tenofovir disoproxil in clinical practice (see section 4.8).
It is recommended that creatinine clearance is calculated in all patients prior to initiating therapy with efavirenz/emtricitabine/tenofovir disoproxil and renal function (creatinine clearance and serum phosphate) is also monitored after two to four weeks of treatment, after three months of treatment and every three to six months thereafter in patients without renal risk factors. In patients with a history of renal dysfunction or in patients who are at risk of renal dysfunction, a more frequent monitoring of renal function is required.
If serum phosphate is <1.5 mg/dl (0.48 mmol/l) or creatinine clearance is decreased to <50 ml/min in any patient receiving efavirenz/emtricitabine/tenofovir disoproxil, renal function must be re-evaluated within one week, including measurements of blood glucose, blood potassium and urine glucose concentrations (see section 4.8, proximal tubulopathy). Since efavirenz/emtricitabine/tenofovir disoproxil is a combination product and the dosing interval of the individual components cannot be altered, treatment with efavirenz/emtricitabine/tenofovir disoproxil must be interrupted in patients with confirmed creatinine clearance < 50 ml/min or decreases in serum phosphate to <1.0 mg/dl (0.32 mmol/l). Interrupting treatment with efavirenz/emtricitabine/tenofovir disoproxil should also be considered in case of progressive decline of renal function when no other cause has been identified. Where discontinuation of therapy with one of the components of efavirenz/emtricitabine/tenofovir disoproxil is indicated or where dose modification is necessary, separate preparations of efavirenz, emtricitabine and tenofovir disoproxil are available.
Bone effects
Bone abnormalities such as osteomalacia which can manifest as persistent or worsening bone pain and, which can infrequently contribute to fractures may be associated with tenofovir disoproxilinduced proximal renal tubulopathy (see section 4.8).
Tenofovir disoproxil may also cause a reduction in bone mineral density (BMD). In HIV infected patients, in a 144-week controlled clinical study (GS-99-903) that compared tenofovir disoproxil with stavudine in combination with lamivudine and efavirenz in antiretroviral-naïve adult patients, small decreases in BMD of the hip and spine were observed in both treatment groups. Decreases in BMD of spine and changes in bone biomarkers from baseline were significantly greater in the tenofovir disoproxil treatment group at 144 weeks. Decreases in BMD of the hip were significantly greater in this group until 96 weeks. However, there was no increased risk of fractures or evidence for clinically relevant bone abnormalities over 144 weeks in this study.
In other studies (prospective and cross-sectional), the most pronounced decreases in BMD were seen in patients treated with tenofovir disoproxil as part of a regimen containing a boosted protease inhibitor. Overall, in view of the bone abnormalities associated with tenofovir disoproxil and the limitations of long-term data on the impact of tenofovir disoproxil on bone health and fracture risk, alternative treatment regimens should be considered for patients with osteoporosis that are at a high risk for fractures.
If bone abnormalities are suspected or detected then appropriate consultation should be obtained.
Skin reactions
Mild-to-moderate rash has been reported with the individual components of efavirenz/emtricitabine/tenofovir disoproxil. The rash associated with the efavirenz component usually resolves with continued therapy. Appropriate antihistamines and/or corticosteroids may improve tolerability and hasten the resolution of rash. Severe rash associated with blistering, moist desquamation or ulceration has been reported in less than 1% of patients treated with efavirenz (see section 4.8). The incidence of erythema multiforme or Stevens-Johnson syndrome was approximately 0.1%. Efavirenz/emtricitabine/tenofovir disoproxil must be discontinued in patients developing severe rash associated with blistering, desquamation, mucosal involvement or fever. Experience with efavirenz in patients who discontinued other antiretroviral agents of the non-nucleoside reverse transcriptase inhibitors (NNRTI) class is limited. Efavirenz/emtricitabine/tenofovir disoproxil is not recommended for patients who have had a life-threatening cutaneous reaction (e.g., Stevens-Johnson syndrome) while taking an NNRTI.
Weight and metabolic parameters
An increase in weight and in levels of blood lipids and glucose may occur during antiretroviral therapy. Such changes may in part be linked to disease control and life style. For lipids, there is in some cases evidence for a treatment effect, while for weight gain there is no strong evidence relating this to any particular treatment. For monitoring of blood lipids and glucose reference is made to established HIV treatment guidelines. Lipid disorders should be managed as clinically appropriate.
Mitochondrial dysfunction following exposure in utero
Nucleos(t)ide analogues may impact mitochondrial function to a variable degree, which is most pronounced with stavudine, didanosine and zidovudine. There have been reports of mitochondrial dysfunction in HIV negative infants exposed in utero and/or postnatally to nucleoside analogues; these have predominantly concerned treatment with regimens containing zidovudine. The main adverse reactions reported are haematological disorders (anaemia, neutropenia) and metabolic disorders (hyperlactataemia, hyperlipasaemia). These events have often been transitory. Late onset neurological disorders have been reported rarely (hypertonia, convulsion, abnormal behaviour). Whether such neurological disorders are transient or permanent is currently unknown. These findings should be considered for any child exposed in utero to nucleos(t)ide analogues, who present with severe clinical findings of unknown etiology, particularly neurologic findings. These findings do not affect current national recommendations to use antiretroviral therapy in pregnant women to prevent vertical transmission of HIV.
Immune reactivation syndrome
In HIV infected patients with severe immune deficiency at the time of institution of CART, an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Typically, such reactions have been observed within the first few weeks or months of initiation of CART. Relevant examples are cytomegalovirus retinitis, generalised and/or focal mycobacterial infections, and Pneumocystis jirovecii pneumonia. Any inflammatory symptoms should be evaluated and treatment instituted when necessary.
Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported to occur in the setting of immune reactivation; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment.
Osteonecrosis
Although the etiology is considered to be multifactorial (including corticosteroid use, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have been reported particularly in patients with advanced HIV disease and/or long-term exposure to CART. Patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Patients with HIV-1 harbouring mutations
Efavirenz/emtricitabine/tenofovir disoproxil should be avoided in patients with HIV-1 harbouring the K65R, M184V/I or K103N mutation (see sections 4.1 and 5.1).
Elderly
Efavirenz/emtricitabine/tenofovir disoproxil has not been studied in patients over the age of 65. Elderly patients are more likely to have decreased hepatic or renal function, therefore caution should be exercised when treating elderly patients with efavirenz/emtricitabine/tenofovir disoproxil (see section 4.2).
Excipients
This medicinal product contains 7.5 mg of sodium metabisulfite per dose, which may rarely cause severe hypersensitivity reactions and bronchospasm.
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, that is to say essentially ‘sodium-free’.
This medicinal product contains 105.5 mg of lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
4.5. Interaction with other medicinal products and other forms of interaction
As Efavirenz/Emtricitabine/Tenofovir disoproxil Mylan contains efavirenz, emtricitabine and tenofovir disoproxil, any interactions that have been identified with these agents individually may occur with Efavirenz/Emtricitabine/Tenofovir disoproxil Mylan. Interaction studies with these agents have only been performed in adults.
As a fixed combination, efavirenz/emtricitabine/tenofovir disoproxil should not be administered concomitantly with other medicinal products containing the components, emtricitabine or tenofovir disoproxil. Efavirenz/emtricitabine/tenofovir disoproxil should not be co-administered with products containing efavirenz unless needed for dose adjustment e.g. with rifampicin (see section 4.2). Due to similarities with emtricitabine, efavirenz/emtricitabine/tenofovir disoproxil should not be administered concomitantly with other cytidine analogues, such as lamivudine. Efavirenz/emtricitabine/tenofovir disoproxil should not be administered concomitantly with adefovir dipivoxil or with medicinal products containing tenofovir alafenamide.
Efavirenz is an in vivo inducer of CYP3A4, CYP2B6 and UGT1A1. Compounds that are substrates of these enzymes may have decreased plasma concentrations when co-administered with efavirenz. Efavirenz may be an inducer of CYP2C19 and CYP2C9; however, inhibition has also been observed in vitro and the net effect of co-administration with substrates of these enzymes is not clear (see section 5.2).
Co-administration of efavirenz/emtricitabine/tenofovir disoproxil with metamizole, which is an inducer of metabolising enzymes including CYP2B6 and CYP3A4 may cause a reduction in plasma concentrations of efavirenz/emtricitabine/tenofovir disoproxil with potential decrease in clinical efficacy. Therefore, caution is advised when metamizole and efavirenz/emtricitabine/tenofovir disoproxil are administered concurrently; clinical response and/or medicinal product levels should be monitored as appropriate.
Efavirenz exposure may be increased when given with medicinal products (for example ritonavir) or food (for example, grapefruit juice) which inhibit CYP3A4 or CYP2B6 activity. Compounds or herbal preparations (for example Ginkgo biloba extracts and St. John’s wort) which induce these enzymes may give rise to decreased plasma concentrations of efavirenz. Concomitant use of St. John’s wort is contraindicated (see section 4.3). Concomitant use of Ginkgo biloba extracts is not recommended (see section 4.4).
In vitro and clinical pharmacokinetic interaction studies have shown the potential for CYP-mediated interactions involving emtricitabine and tenofovir disoproxil with other medicinal products is low.
Cannabinoid test interaction
Efavirenz does not bind to cannabinoid receptors. False-positive urine cannabinoid test results have been reported with some screening assays in uninfected and HIV infected subjects receiving efavirenz. Confirmatory testing by a more specific method such as gas chromatography/mass spectrometry is recommended in such cases.
Contraindications of concomitant use
Efavirenz/emtricitabine/tenofovir disoproxil must not be administered concurrently with terfenadine, astemizole, cisapride, midazolam, triazolam, pimozide, bepridil, or ergot alkaloids (for example, ergotamine, dihydroergotamine, ergonovine, and methylergonovine), since inhibition of their metabolism may lead to serious, life-threatening events (see section 4.3).
Elbasvir/grazoprevir
Co administration of efavirenz/emtricitabine/tenofovir disoproxil with elbasvir/grazoprevir is contraindicated because it may lead to loss of virologic response to elbasvir/grazoprevir (see section 4.3 and Table 1).
Voriconazole
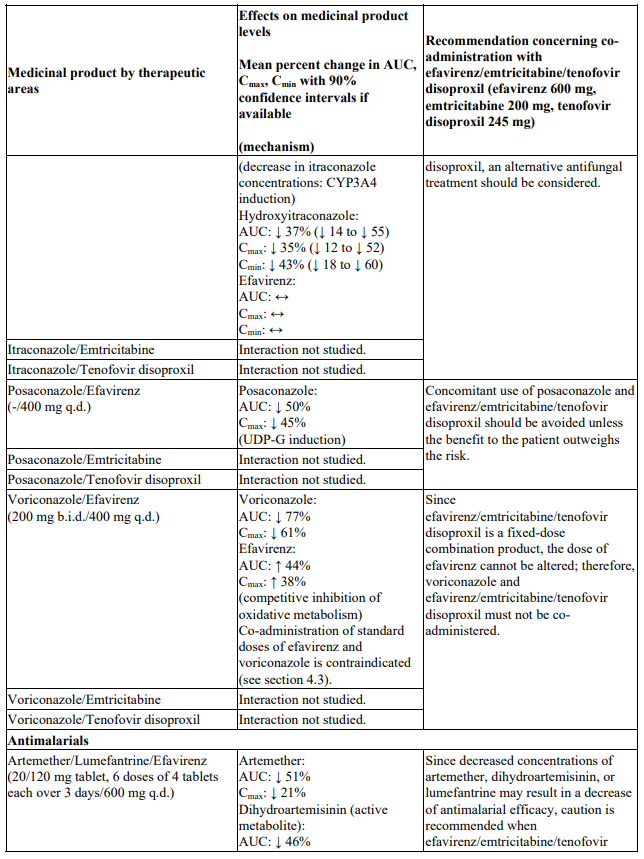
Co-administration of standard doses of efavirenz and voriconazole is contraindicated. Since efavirenz/emtricitabine/tenofovir disoproxil is a fixed-dose combination product, the dose of efavirenz cannot be altered; therefore, voriconazole and efavirenz/emtricitabine/tenofovir disoproxil must not be co-administered (see section 4.3 and Table 1).
St. John’s wort (Hypericum perforatum)
Co-administration of efavirenz/emtricitabine/tenofovir disoproxil and St. John’s wort or herbal preparations containing St. John’s wort is contraindicated. Plasma levels of efavirenz can be reduced by concomitant use of St. John’s wort due to induction of medicinal product metabolising enzymes and/or transport proteins by St. John’s wort. If a patient is already taking St. John’s wort, stop St. John’s wort, check viral levels and if possible efavirenz levels. Efavirenz levels may increase on stopping St. John’s wort. The inducing effect of St. John’s wort may persist for at least 2 weeks after cessation of treatment (see section 4.3).
QT Prolonging medicinal products
Efavirenz/emtricitabine/tenofovir disoproxil is contraindicated with concomitant use of medicinal products that are known to prolong the QTc interval and could lead to Torsade de Pointes, such as: antiarrhythmics of classes IA and III, neuroleptics and antidepressant agents, certain antibiotics including some agents of the following classes: macrolides, fluoroquinolones, imidazole, and triazole antifungal agents, certain non-sedating antihistaminics (terfenadine, astemizole), cisapride, flecainide, certain antimalarials and methadone (see section 4.3).
Concomitant use not recommended
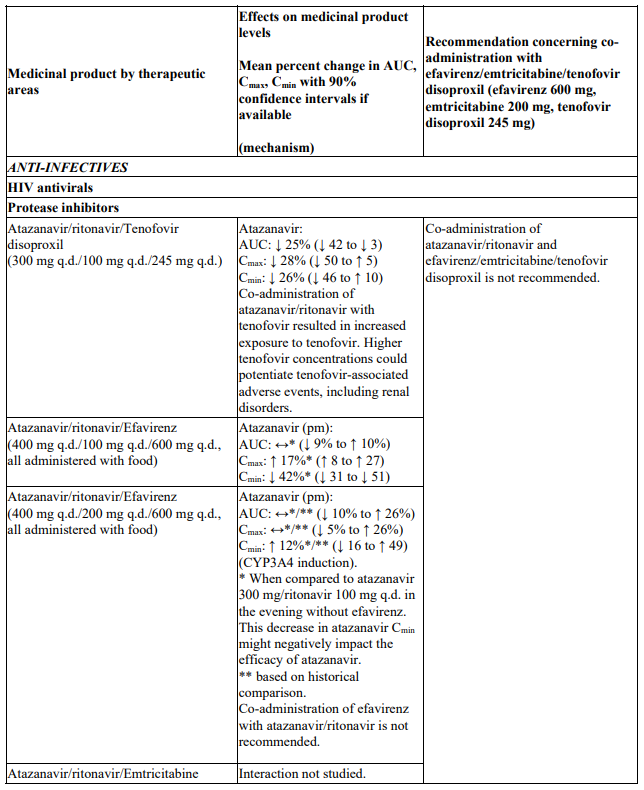
Atazanavir/ritonavir
Insufficient data are available to make a dosing recommendation for atazanavir/ritonavir in combination with efavirenz/emtricitabine/tenofovir disoproxil. Therefore coadministration of atazanavir/ritonavir and efavirenz/emtricitabine/tenofovir disoproxil is not recommended (see Table 1).
Didanosine
Co-administration of efavirenz/emtricitabine/tenofovir disoproxil and didanosine is not recommended (see Table 1). Sofosbuvir/velpatasvir and sofosbuvir/velpatasvir/voxilaprevir: Co-administration of efavirenz/emtricitabine/tenofovir disoproxil and sofosbuvir/velpatasvir or sofosbuvir/velpatasvir/voxilaprevir is not recommended (see section 4.4 and Table 1).
Praziquantel
Concomitant use of efavirenz with praziquantel is not recommended due to significant decrease in plasma concentrations of praziquantel, with risk of treatment failure due to increased hepatic metabolism by efavirenz. In case the combination is needed, an increased dose of praziquantel could be considered.
Renally eliminated medicinal products
Since emtricitabine and tenofovir are primarily eliminated by the kidneys, co-administration of efavirenz/emtricitabine/tenofovir disoproxil with medicinal products that reduce renal function or compete for active tubular secretion (e.g. cidofovir) may increase serum concentrations of emtricitabine, tenofovir and/or the co-administered medicinal products.
Use of efavirenz/emtricitabine/tenofovir disoproxil should be avoided with concurrent or recent use of a nephrotoxic medicinal product. Some examples include, but are not limited to, aminoglycosides, amphotericin B, foscarnet, ganciclovir, pentamidine, vancomycin, cidofovir or interleukin-2 (see section 4.4).
Other interactions
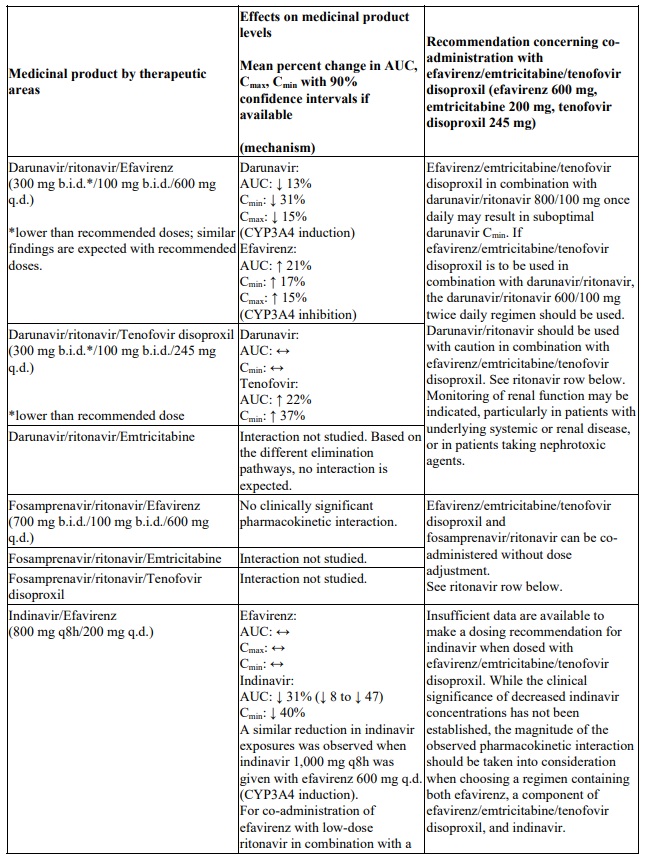
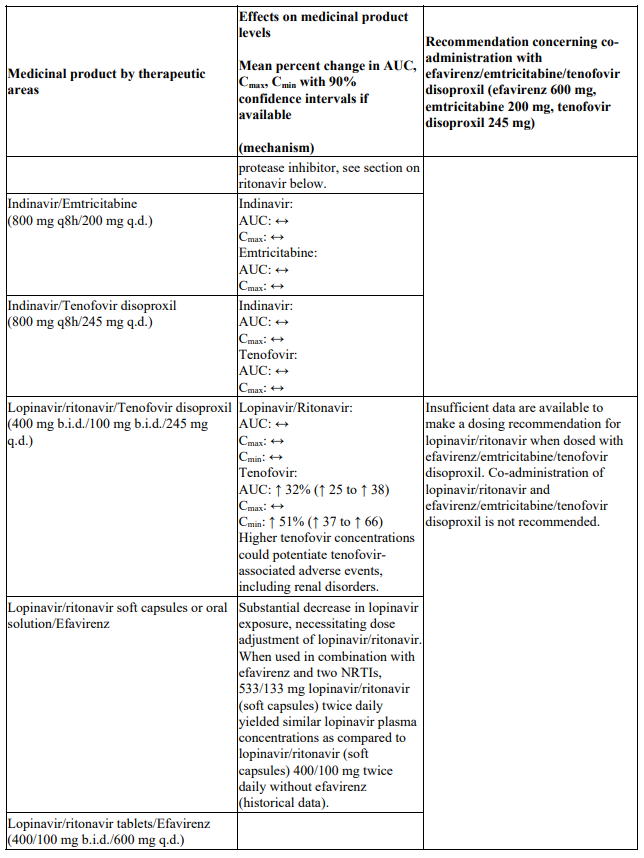
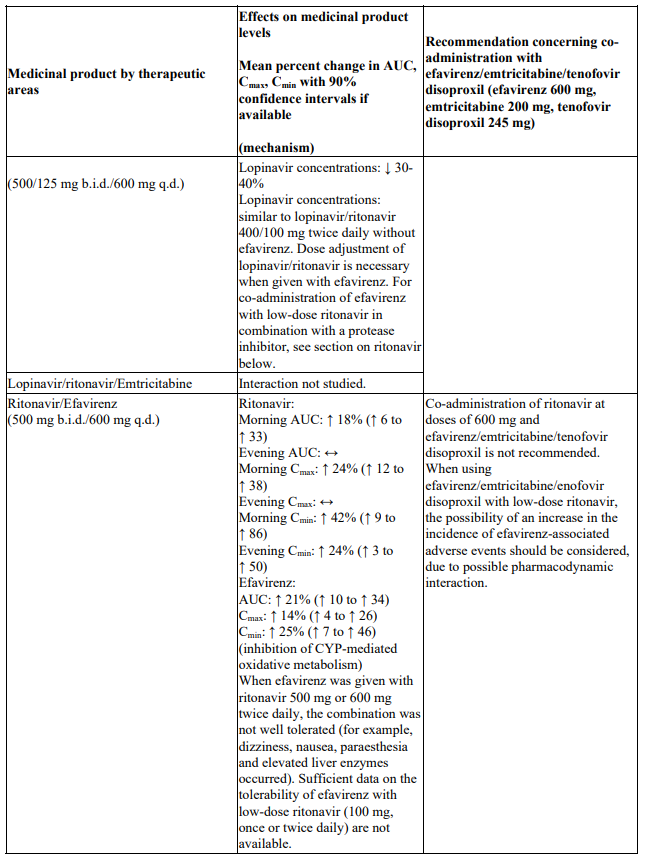
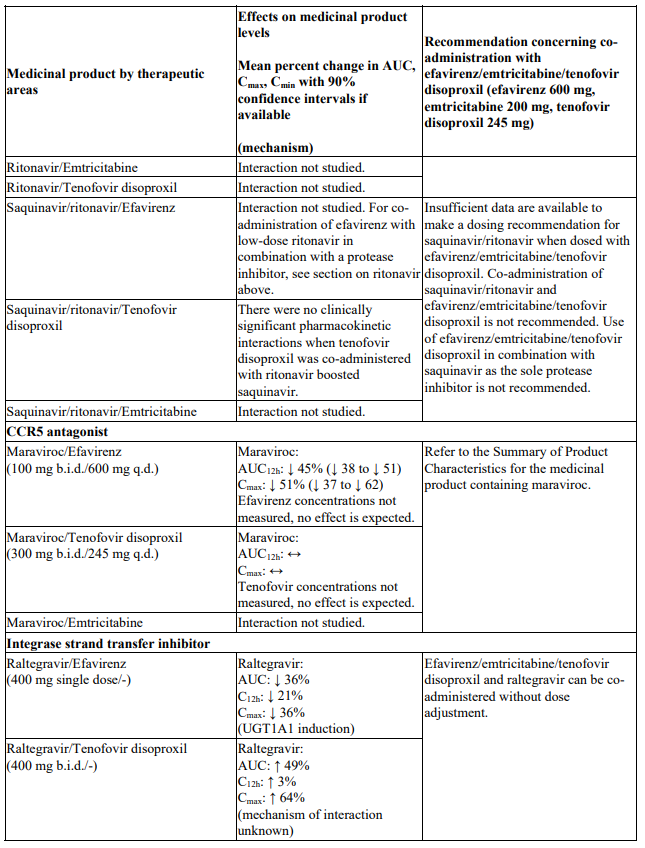
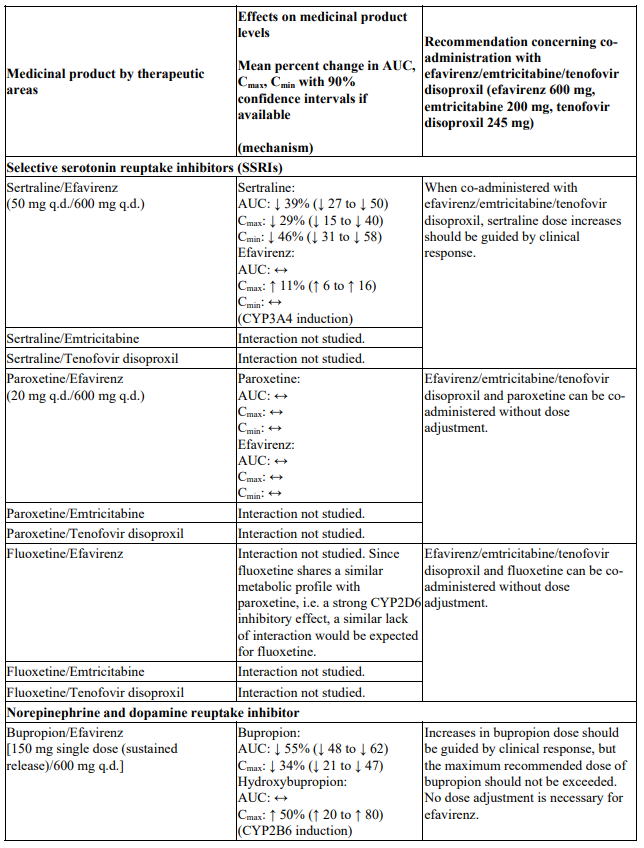
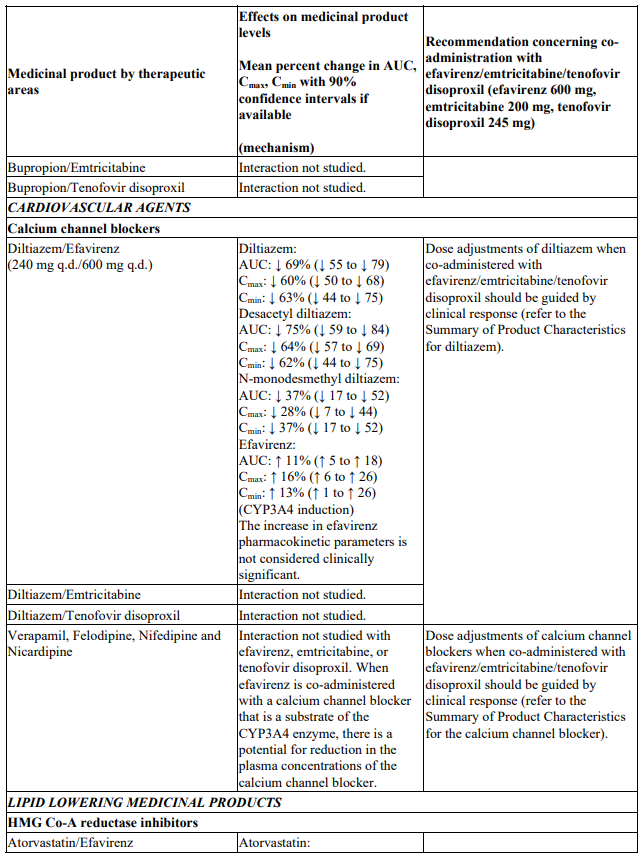
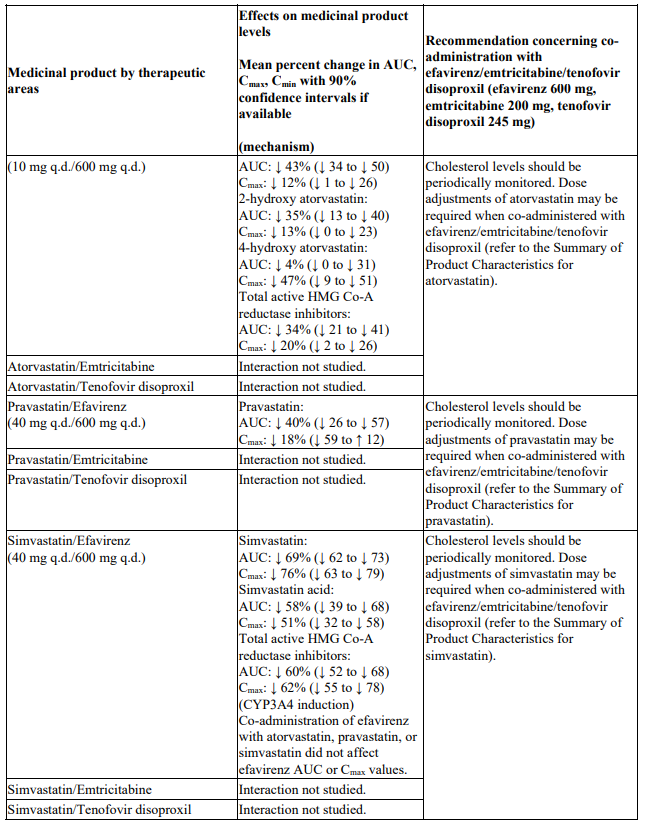
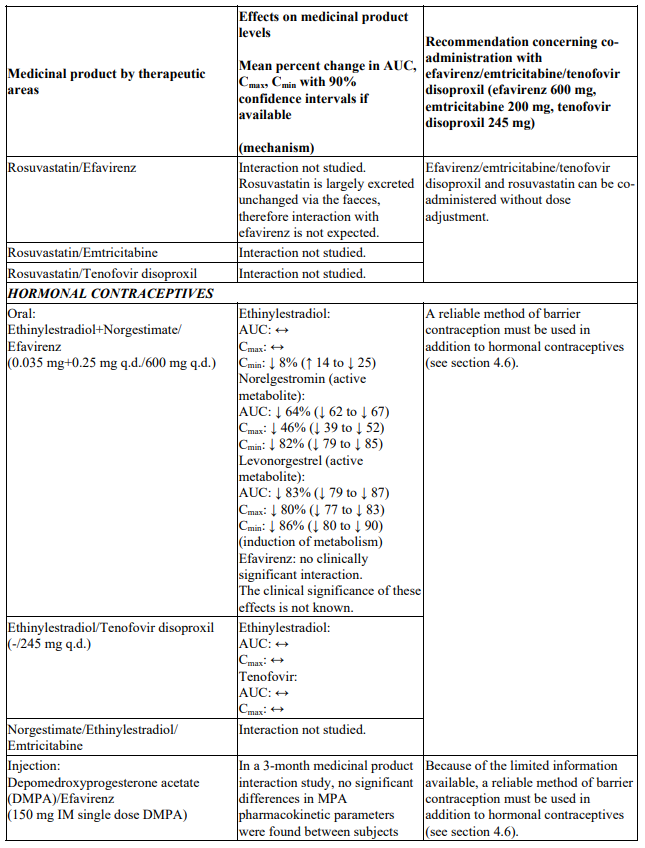
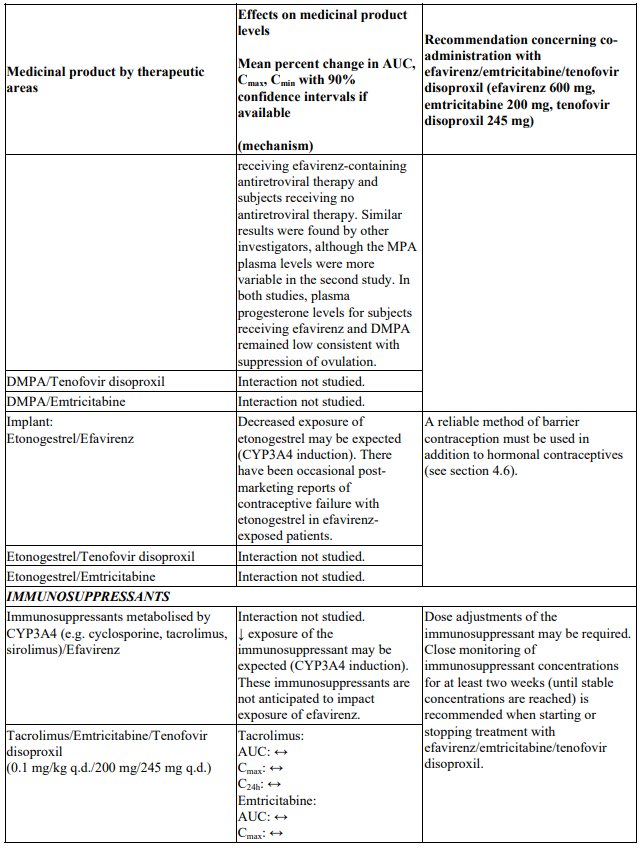
Interactions between efavirenz/emtricitabine/tenofovir disoproxil or its individual component(s) and other medicinal products are listed in Table 1 below (increase is indicated as ‘↑’, decrease as ‘↓’, no change as ‘↔’, twice daily as ‘b.i.d.’, once daily as ‘q.d.’ and once every 8 hours as ‘q8h’). If available, 90% confidence intervals are shown in parentheses.
Table 1. Interactions between efavirenz/emtricitabine/tenofovir disoproxil or its individual components and other medicinal products:
Studies conducted with other medicinal products
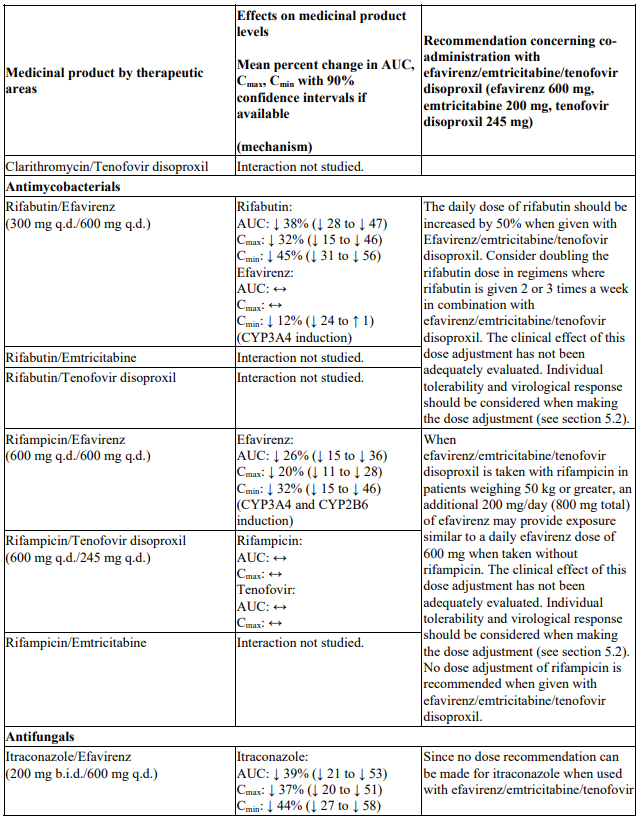
There were no clinically significant pharmacokinetic interactions when efavirenz was administered with azithromycin, cetirizine, fosamprenavir/ritonavir, lorazepam, zidovudine, aluminium/magnesium hydroxide antacids, famotidine or fluconazole. The potential for interactions with efavirenz and other azole antifungals, such as ketoconazole, has not been studied.
There were no clinically significant pharmacokinetic interactions when emtricitabine was administered with stavudine, zidovudine or famciclovir. There were no clinically significant pharmacokinetic interactions when tenofovir disoproxil was co-administered with emtricitabine, or ribavirin.
4.6. Fertility, pregnancy and lactation
Women of childbearing potential (see below and section 5.3)
Pregnancy should be avoided in women receiving efavirenz/emtricitabine/tenofovir disoproxil. Women of childbearing potential should undergo pregnancy testing before initiation of efavirenz/emtricitabine/tenofovir disoproxil.
Contraception in males and females
Barrier contraception should always be used in combination with other methods of contraception (for example, oral or other hormonal contraceptives, see section 4.5) while on therapy with efavirenz/emtricitabine/tenofovir disoproxil. Because of the long half-life of efavirenz, use of adequate contraceptive measures for 12 weeks after discontinuation of efavirenz/emtricitabine/tenofovir disoproxil is recommended.
Pregnancy
Efavirenz: There have been seven retrospective reports of findings consistent with neural tube defects, including meningomyelocele, all in mothers exposed to efavirenz-containing regimens (excluding any efavirenz-containing fixed-dose combination tablets) in the first trimester. Two additional cases (1 prospective and 1 retrospective) including events consistent with neural tube defects have been reported with the fixed-dose combination tablet containing efavirenz, emtricitabine, and tenofovir disoproxil. A causal relationship of these events to the use of efavirenz has not been established, and the denominator is unknown. As neural tube defects occur within the first 4 weeks of foetal development (at which time neural tubes are sealed), this potential risk would concern women exposed to efavirenz during the first trimester of pregnancy.
As of July 2013, the Antiretroviral Pregnancy Registry (APR) has received prospective reports of 904 pregnancies with first trimester exposure to efavirenz-containing regimens, resulting in 766 live births. One child was reported to have a neural tube defect, and the frequency and pattern of other birth defects were similar to those seen in children exposed to non-efavirenz-containing regimens, as well as those in HIV negative controls. The incidence of neural tube defects in the general population ranges from 0.5-1 case per 1,000 live births.
Malformations have been observed in foetuses from efavirenz-treated monkeys (see section 5.3).
Emtricitabine and tenofovir disoproxil: A large amount of data on pregnant women (more than 1,000 pregnancy outcomes) indicates no malformations or foetal/neonatal toxicity associated with emtricitabine and tenofovir disoproxil. Animal studies on emtricitabine and tenofovir disoproxil do not indicate reproductive toxicity (see section 5.3).
Efavirenz/emtricitabine/tenofovir disoproxil should not be used during pregnancy unless the clinical condition of the woman requires treatment with efavirenz/emtricitabine/tenofovir disoproxil.
Breast-feeding
Efavirenz, emtricitabine and tenofovir have been shown to be excreted in human milk. There is insufficient information on the effects of efavirenz, emtricitabine and tenofovir in newborns/infants. A risk to the infants cannot be excluded. Therefore efavirenz/emtricitabine/tenofovir disoproxil should not be used during breast-feeding.
It is recommended that women living with HIV do not breast-feed their infants in order to avoid transmission of HIV.
Fertility
No human data on the effect of efavirenz/emtricitabine/tenofovir disoproxil are available. Animal studies do not indicate harmful effects of efavirenz, emtricitabine or tenofovir disoproxil on fertility.
4.7. Effects on ability to drive and use machines
No studies on the effects on the ability to drive and use machines have been performed. However, dizziness has been reported during treatment with efavirenz, emtricitabine and tenofovir disoproxil. Efavirenz may also cause impaired concentration and/or somnolence. Patients should be instructed that if they experience these symptoms they should avoid potentially hazardous tasks such as driving and operating machinery.
4.8. Undesirable effects
Summary of the safety profile
The combination of efavirenz, emtricitabine and tenofovir disoproxil has been studied in 460 patients either as the fixed-dose combination tablet efavirenz/emtricitabine/tenofovir disoproxil (study AI266073) or as the component products (study GS-01-934). Adverse reactions were generally consistent with those seen in previous studies of the individual components. The most frequently reported adverse reactions considered possibly or probably related to efavirenz/emtricitabine/tenofovir disoproxil among patients treated up to 48 weeks in study AI266073 were psychiatric disorders (16%), nervous system disorders (13%), and gastrointestinal disorders (7%).
Severe skin reactions such as Stevens-Johnson syndrome and erythema multiforme; neuropsychiatric adverse reactions (including severe depression, death by suicide, psychosis-like behaviour, seizures); severe hepatic events; pancreatitis and lactic acidosis (sometimes fatal) have been reported.
Rare events of renal impairment, renal failure and uncommon events of proximal renal tubulopathy (including Fanconi syndrome) sometimes leading to bone abnormalities (infrequently contributing to fractures) have also been reported. Monitoring of renal function is recommended for patients receiving efavirenz/emtricitabine/tenofovir disoproxil (see section 4.4).
Discontinuation of efavirenz/emtricitabine/tenofovir disoproxil therapy in patients co-infected with HIV and HBV may be associated with severe acute exacerbations of hepatitis (see section 4.4).
The administration of efavirenz/emtricitabine/tenofovir disoproxil with food may increase efavirenz exposure and may lead to an increase in the frequency of adverse reactions (see sections 4.4 and 5.2).
Tabulated list of adverse reactions
The adverse reactions from clinical study and post-marketing experience with efavirenz/emtricitabine/tenofovir disoproxil and the individual components of efavirenz/emtricitabine/tenofovir disoproxil in antiretroviral combination therapy are listed in Table 2 below by body system organ class, frequency and the component(s) of efavirenz/emtricitabine/tenofovir disoproxil to which the adverse reactions are attributable. Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. Frequencies are defined as very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100) or rare (≥1/10,000 to <1/1,000).
Adverse reactions associated with the use of efavirenz/emtricitabine/tenofovir disoproxil
Treatment-emergent adverse reactions considered possibly or probably related to efavirenz/emtricitabine/tenofovir disoproxil reported in study AI266073 (over 48 weeks; n=203), which have not been associated with one of the individual components of efavirenz/emtricitabine/tenofovir disoproxil, include:
Common: anorexia
Uncommon: dry mouth, incoherent speech, increased appetite, libido decreased, myalgia
Table 2. Adverse reactions associated with efavirenz/emtricitabine/tenofovir disoproxil listed by the component(s) of efavirenz/emtricitabine/tenofovir disoproxil to which the adverse reactions are attributable:
| Efavirenz/emtricitabine/tenofovir disoproxil | |||
|---|---|---|---|
| Efavirenz | Emtricitabine | Tenofovir disoproxil | |
| Blood and lymphatic system disorders | |||
| Common | neutropenia | ||
| Uncommon | anaemia1 | ||
| Immune system disorders | |||
| Common | allergic reaction | ||
| Uncommon | hypersensitivity | ||
| Metabolism and nutrition disorders | |||
| Very common | hypophosphataemia2 | ||
| Common | hypertriglyceridaemia3 | hyperglycaemia, hypertriglyceridaemia | |
| Uncommon | hypercholesterolaemia3 | hypokalaemia2 | |
| Rare | lactic acidosis | ||
| Psychiatric disorders | |||
| Common | depression (severe in 1.6%)3, anxiety3, abnormal dreams3, insomnia3 abnormal dreams, insomnia | abnormal dreams, insomnia | |
| Uncommon | suicide attempt3, suicide ideation3, psychosis3, mania3, paranoia3, hallucination3, euphoric mood3, affect lability3, confusional state3, aggression3, catatonia3 | ||
| Rare | completed suicide3,4, delusion3,4, neurosis3,4 | ||
| Nervous system disorders | |||
| Very common | headache | dizziness | |
| Common | cerebellar coordination and balance disturbances3, somnolence (2.0%)3, headache (5.7%)3, disturbance in attention (3.6%)3, dizziness (8.5%)3 | dizziness | headache |
| Uncommon | convulsions3, amnesia3, thinking abnormal3, ataxia3, coordination abnormal3, agitation3, tremor | ||
| Eye disorders | |||
| Uncommon | vision blurred | ||
| Ear and labyrinth disorders | |||
| Uncommon | tinnitus, vertigo | ||
| Vascular disorders | |||
| Uncommon | flushing | ||
| Gastrointestinal disorders | |||
| Very common | diarrhoea, nausea | diarrhoea, vomiting, nausea | |
| Common | diarrhoea, vomiting, abdominal pain, nausea | elevated amylase including elevated pancreatic amylase, elevated serum lipase, vomiting, abdominal pain, dyspepsia | abdominal pain, abdominal distension, flatulence |
| Uncommon | pancreatitis | pancreatitis | |
| Hepatobiliary disorders | |||
| Common | elevated aspartate aminotransferase (AST), elevated alanine aminotransferase (ALT), elevated gamma- glutamyltransferase (GGT) | elevated serum AST and/or elevated serum ALT, hyperbilirubinaemia | increased transaminases |
| Uncommon | hepatitis acute | ||
| Rare | hepatic failure3,4 | hepatic steatosis, hepatitis | |
| Skin and subcutaneous tissue disorders | |||
| Very common | rash (moderate-severe, 11.6%, all grades, 18%)3 | rash | |
| Common | pruritus | vesiculobullous rash, pustular rash, maculopapular rash, rash, pruritus, urticaria, skin discolouration (increased pigmentation)1 | |
| Uncommon | Stevens-Johnson syndrome, erythema multiforme3, severe rash (<1%) | angioedema4 | |
| Rare | photoallergic dermatitis | angioedema | |
| Musculoskeletal and connective tissue disorders | |||
| Very common | elevated creatine kinase | ||
| Uncommon | rhabdomyolysis2, muscular weakness2 | ||
| Rare | osteomalacia (manifested as bone pain and infrequently contributing to fractures)2,4, myopathy2 | ||
| Renal and urinary disorders | |||
| Uncommon | increased creatinine, proteinuria, proximal renal tubulopathy including Fanconi syndrome | ||
| Rare | renal failure (acute and chronic), acute tubular necrosis, nephritis (including acute interstitial nephritis)4, nephrogenic diabetes insipidus | ||
| Reproductive system and breast disorders | |||
| Uncommon | gynaecomastia | ||
| General disorders and administration site conditions | |||
| Very common | asthenia | ||
| Common | fatigue | pain, asthenia | |
1 Anaemia was common and skin discolouration (increased pigmentation) was very common when emtricitabine was administered to paediatric patients.
2 This adverse reaction may occur as a consequence of proximal renal tubulopathy. It is not considered to be causally associated with tenofovir disoproxil in the absence of this condition.
3 See section 4.8 Description of selected adverse reactions for more details.
4 This adverse reaction was identified through post-marketing surveillance for either efavirenz, emtricitabine or tenofovir disoproxil. The frequency category was estimated from a statistical calculation based on the total number of patients treated with efavirenz in clinical studies (n=3,969) or exposed to emtricitabine in randomised controlled clinical studies (n=1,563) or exposed to tenofovir disoproxil in randomised controlled clinical studies and the expanded access programme (n=7,319).
Description of selected adverse reactions
Rash
In clinical studies of efavirenz, rashes were usually mild-to-moderate maculopapular skin eruptions that occurred within the first two weeks of initiating therapy with efavirenz. In most patients rash resolved with continuing therapy with efavirenz within one month. Efavirenz/emtricitabine/tenofovir disoproxil can be reinitiated in patients interrupting therapy because of rash. Use of appropriate antihistamines and/or corticosteroids is recommended when efavirenz/emtricitabine/tenofovir disoproxil is restarted.
Psychiatric symptoms
Patients with a history of psychiatric disorders appear to be at greater risk of serious psychiatric adverse reactions listed in the efavirenz column of Table 2.
Nervous system symptoms
Nervous system symptoms are common with efavirenz, one of the components of efavirenz/emtricitabine/tenofovir disoproxil. In clinical controlled studies of efavirenz, nervous system symptoms of moderate to severe intensity were experienced by 19% (severe 2%) of patients, and 2% of patients discontinued therapy due to such symptoms. They usually begin during the first one or two days of efavirenz therapy and generally resolve after the first two to four weeks. They may occur more frequently when efavirenz/emtricitabine/tenofovir disoproxil is taken concomitantly with meals possibly due to increased efavirenz plasma levels (see section 5.2). Dosing at bedtime seems to improve the tolerability of these symptoms (see section 4.2).
Hepatic failure with efavirenz
Hepatic failure, including cases in patients with no pre-existing hepatic disease or other identifiable risk factors, as reported post-marketing, were sometimes characterised by a fulminant course, progressing in some cases to transplantation or death.
Renal impairment
As efavirenz/emtricitabine/tenofovir disoproxil may cause renal damage, monitoring of renal function is recommended (see sections 4.4 and 4.8 Summary of the safety profile). Proximal renal tubulopathy generally resolved or improved after tenofovir disoproxil discontinuation. However, in some patients, declines in creatinine clearance did not completely resolve despite tenofovir disoproxil discontinuation. Patients at risk of renal impairment (such as patients with baseline renal risk factors, advanced HIV disease, or patients receiving concomitant nephrotoxic medicinal products) are at increased risk of experiencing incomplete recovery of renal function despite tenofovir disoproxil discontinuation (see section 4.4).
Lactic acidosis
Cases of lactic acidosis have been reported with tenofovir disoproxil alone or in combination with other antiretrovirals. Patients with predisposing factors such as severe hepatic impairment (CPT, Class C) (see section 4.3), or patients receiving concomitant medicinal products known to induce lactic acidosis are at increased risk of experiencing severe lactic acidosis during tenofovir disoproxil treatment, including fatal outcomes.
Metabolic parameters
Weight and levels of blood lipids and glucose may increase during antiretroviral therapy (see section 4.4).
Immune Reactivation Syndrome
In HIV infected patients with severe immune deficiency at the time of initiation of CART, an inflammatory reaction to asymptomatic or residual opportunistic infections may arise. Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment (see section 4.4).
Osteonecrosis
Cases of osteonecrosis have been reported, particularly in patients with generally acknowledged risk factors, advanced HIV disease or long-term exposure to CART. The frequency of this is unknown (see section 4.4).
Paediatric population
Insufficient safety data are available for children below 18 years of age.
Efavirenz/emtricitabine/tenofovir disoproxil is not recommended in this population (see section 4.2).
Other special populations
Elderly
Efavirenz/emtricitabine/tenofovir disoproxil has not been studied in patients over the age of 65. Elderly patients are more likely to have decreased hepatic or renal function, therefore caution should be exercised when treating elderly patients with efavirenz/emtricitabine/tenofovir disoproxil (see section 4.2).
Patients with renal impairment
Since tenofovir disoproxil can cause renal toxicity, close monitoring of renal function is recommended in any patient with mild renal impairment treated with efavirenz/emtricitabine/tenofovir disoproxil (see sections 4.2, 4.4 and 5.2).
HIV/HBV or HCV co-infected patients
Only a limited number of patients were co-infected with HBV (n=13) or HCV (n=26) in study GS-01-934. The adverse reaction profile of efavirenz, emtricitabine and tenofovir disoproxil in patients co-infected with HIV/HBV or HIV/HCV was similar to that observed in patients infected with HIV without co-infection. However, as would be expected in this patient population, elevations in AST and ALT occurred more frequently than in the general HIV infected population.
Exacerbations of hepatitis after discontinuation of treatment
In HIV infected patients co-infected with HBV, clinical and laboratory evidence of hepatitis may occur after discontinuation of treatment (see section 4.4).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.