EFRIN Film-coated tablet Ref.[51182] Active ingredients: Efavirenz
Source: Health Products Regulatory Authority (ZA) Revision Year: 2023 Publisher: Viatris Healthcare (Pty) Ltd, 4 Brewery Street, Isando, Johannesburg, 1600, Gauteng, South Africa
4.3. Contraindications
- EFRIN is contraindicated in patients with hypersensitivity to efavirenz or any of the excipients of EFRIN.
- EFRIN should not be administered concurrently with cisapride, midazolam, triazolam or ergot derivatives because competition for CYP3A4 by efavirenz could result in inhibition of metabolism of these medicines and create the potential for serious and/or life-threatening adverse events (e.g. cardiac dysrhythmias, prolonged sedation or respiratory depression).
- Pregnancy and lactation (see section 4.6).
- EFRIN is contraindicated in adults and children weighing less than 40 kg.
- A history of previous liver injury/failure with efavirenz containing antiretroviral treatment (ART).
- Patients with severe hepatic impairment (Child Pugh Grade C) (see section 5.2).
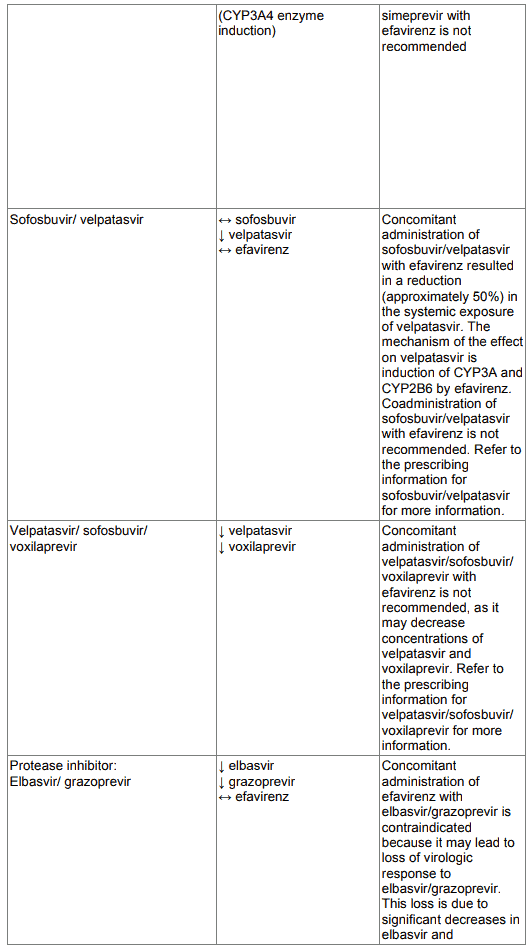
- Co-administration with elbasvir (EBR) and grazoprevir (GZR) due to the potential for significant decreases in plasma concentrations of EBR and GZR (see section 4.5).
- Herbal preparations containing St. John’s wort (Hypericum perforatum) due to the risk of decreased plasma concentrations and reduced clinical effects of efavirenz (see section 4.5).
- Patients with:
- a family history of sudden death or of congenital prolongation of the QTc interval on electrocardiograms, or with any other clinical condition known to prolong the QTc interval.
- a history of symptomatic cardiac dysrhythmia or with clinically relevant bradycardia or with congestive cardiac failure accompanied by reduced left ventricle ejection fraction.
- severe disturbances of electrolyte balance e.g. hypokalemia or hypomagnesemia.
- Patients taking medicines that are known to prolong the QTc interval (prodysrhythmic).
These medicines include:- antidysrhythmic of classes IA and III,
- neuroleptics, antidepressive medicines,
- certain antibiotics including some medicines of the following classes: macrolides, fluoroquinolones, imidazole and triazole antifungal medicines,
- certain non-sedating antihistamines (terfenadine),
- cisapride,
- flecainide,
- certain antimalarials,
- methadone.
4.4. Special warnings and precautions for use
Resistant human immuno-virus (HIV) strains emerge rapidly when EFRIN is administered as monotherapy, therefore, EFRIN must not be used as a single medicine to treat HIV or added on as a sole medicine to a failing regimen. When prescribing medications concomitantly with EFRIN, medical practitioners should refer to the corresponding manufacturer’s medicine package insert.
If any antiretroviral medication in a combination regimen is interrupted because of e.g. suspected intolerance, serious consideration should be given to simultaneous discontinuation of all antiretroviral medications. The antiretroviral medications should be restarted at the same time upon resolution of the intolerance symptoms. Intermittent monotherapy and sequential reintroduction of antiretroviral medicines is not advisable because of the increased potential for selection of medicine-resistant mutant virus.
Co-administration of efavirenz as contained in EFRIN with the fixed combination tablet containing efavirenz, emtricitabine, and tenofovir disoproxil is not recommended unless needed for dose adjustment (for example, with rifampicin).
Co-administration of sofosbuvir/velpatasvir with efavirenz is not recommended (see section 4.5). Concomitant administration of velpatasvir/sofosbuvir/ voxilaprevir with EFRIN is not recommended (see section 4.5).
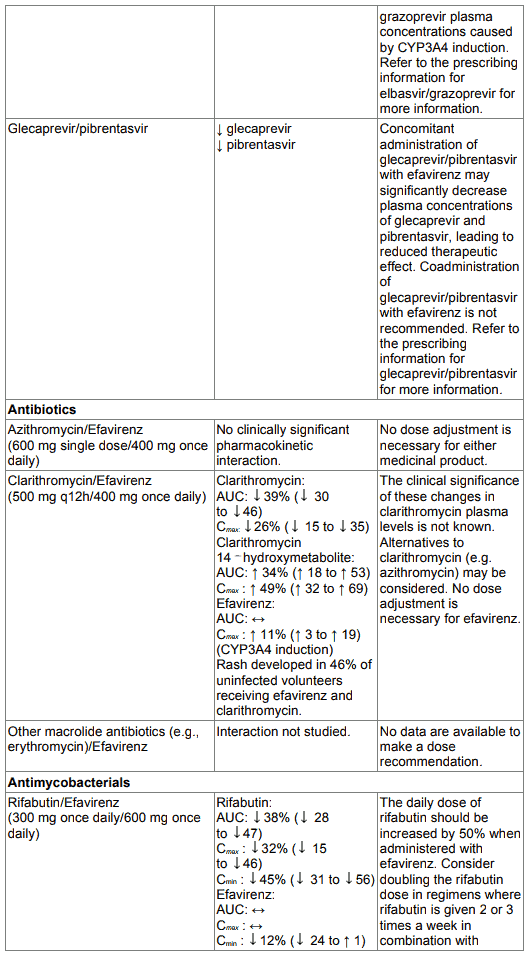
Co-administration of glecaprevir/pibrentasvir with efavirenz may significantly decrease plasma concentrations of glecaprevir and pibrentasvir, leading to reduced therapeutic effect. Coadministration of glecaprevir/pibrentasvir with efavirenz is not recommended (see section 4.5).
Concomitant use of Ginkgo biloba extracts is not recommended (see section 4.5).
While effective viral suppression with antiretroviral therapy has been proven to substantially reduce the risk of sexual transmission, a residual risk cannot be excluded. Precautions to prevent transmission should be taken.
Skin Rash
Mild-to-moderate rash has been reported with EFRIN use and usually resolves with continued therapy. Appropriate antihistamines and/or corticosteroids may improve the tolerability and hasten the resolution of rash. EFRIN should be discontinued in patients developing severe rash associated with blistering, desquamation, mucosal involvement or fever. If therapy with EFRIN is discontinued, consideration should also be given to interrupting therapy with other antiretroviral medicine to avoid development of resistant virus (see section 4.8).
Prophylaxis with appropriate antihistamines prior to initiating therapy with EFRIN in children may be considered.
EFRIN is not recommended for patients who have had a life-threatening cutaneous reaction (e.g., Stevens-Johnson syndrome) while taking another NNRTI.
Psychiatric symptoms
Psychiatric adverse reactions have been reported in patients treated with EFRIN. Patients with a prior history of psychiatric disorders appear to be at greater risk of these serious psychiatric adverse reactions. In particular, severe depression was more common in those with a history of depression. There have also been post-marketing reports of severe depression, death by suicide, delusions, psychosis-like behaviour and catatonia. Patients should be advised that if they experience symptoms such as severe depression, psychosis or suicidal ideation, they should contact their doctor immediately to assess the possibility that the symptoms may be related to the use of EFRIN, and if so, to determine whether the risks of continued therapy outweigh the benefits (see section 4.8).
Nervous System Symptoms
Nervous system symptoms have been reported with EFRIN use (see section 4.8). In addition, there have been reports of inappropriate behaviour (including aggressive reactions), predominantly in patients with a history of mental illness or substance abuse.
Seizures
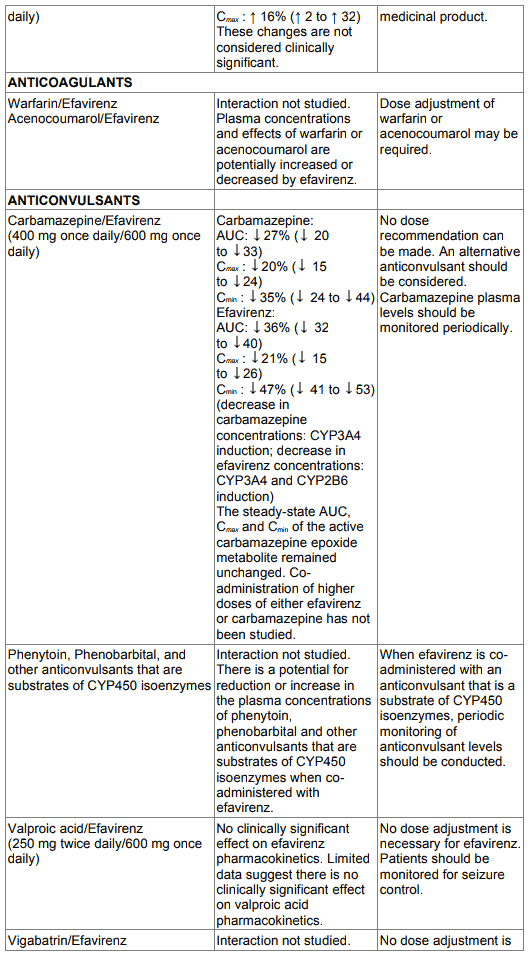
Convulsions have been observed in adult and paediatric patients receiving EFRIN, generally in the presence of known medical history of seizures. Patients who are receiving concomitant anticonvulsant medicinal products primarily metabolised by the liver, such as phenytoin, carbamazepine and phenobarbital, may require periodic monitoring of plasma levels. In a medicine interaction study, carbamazepine plasma concentrations were decreased when carbamazepine was coadministered with efavirenz as contained in EFRIN (see section 4.5). Caution must be taken in any patient with a history of seizures.
QTc Prolongation
QTc prolongation has been observed with the use of EFRIN (see sections 4.5 and 5.1). Consider alternatives to efavirenz for coadministration with a medicine with a known risk of Torsade de Pointes or when to be administered to patients at higher risk of Torsade de Pointes.
Effect of food
The administration of EFRIN with food may increase efavirenz exposure (see section 5.2) and may lead to an increase in the frequency of adverse reactions (see section 4.8). It is recommended that EFRIN be taken on an empty stomach, preferably at bedtime.
Weight and metabolic parameters
An increase in weight and in levels of blood lipids and glucose may occur during antiretroviral therapy. Such changes may in part be linked to disease control and lifestyle. For monitoring of blood lipids and glucose reference is made to established HIV treatment guidelines. Lipid disorders should be managed as clinically appropriate.
Lipodystrophy and metabolic abnormalities
Combination antiretroviral therapy has been associated with the redistribution/accumulation of body fat, including central obesity, dorso-cervical fat, enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and elevated serum lipid and glucose levels in HIV patients. Clinical examination should include evaluation for physical signs of fat redistribution. Patients with evidence of lipodystrophy should have a thorough cardiovascular risk assessment. Immune Reconstitution Inflammatory Syndrome: Immune reconstitution inflammatory syndrome (IRIS) is an immunopathological response resulting from the rapid restoration of pathogen-specific immune responses to pre-existing antigens combined with immune dysregulation, which occurs shortly after starting combination Anti-Retroviral Therapy (cART). Typically such reaction presents by paradoxical deterioration of opportunistic infections being treated or with unmasking of an asymptomatic opportunistic disease, often with an atypical inflammatory presentation. IRIS usually develops within the first three months of initiation of ART and occurs more commonly in patients with low CD4 counts.
Common examples of IRIS reactions to opportunistic diseases are tuberculosis, cytomegalovirus retinitis, and cryptococcal meningitis. Appropriate treatment of the opportunistic disease should be instituted or continued and ART continued. Inflammatory manifestations generally subside after a few weeks. Severe cases may respond to glucocorticoids, but there is only limited evidence for this in patients with tuberculosis IRIS. Autoimmune disorders (such as Graves' disease) have also been reported as IRIS reactions; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment.
Osteonecrosis
Although the aetiology is considered to be multifactorial (including corticosteroid use, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have been reported, particularly in patients with advanced HIV-disease and/or long-term exposure to combination antiretroviral therapy (cART). Patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Opportunistic infections
Patients receiving EFRIN should be advised that they may continue to develop opportunistic infections and other complications of HIV infection, and therefore they should remain under close observation by healthcare professionals experienced in the treatment of patients with associated HIV disease. Regular monitoring of viral load and CD4 counts needs to be done.
The risk of HIV transmission to others
Patients should be advised that current antiretroviral therapy, including EFRIN, does not prevent the risk of transmission of HIV to others through sexual contact or blood contamination. Appropriate precautions should continue to be employed.
Cholesterol
Monitoring of cholesterol and triglycerides should be considered in patients treated with EFRIN (see section 4.8).
Special Populations
Because of the extensive cytochrome P450-mediated metabolism of EFRIN and limited clinical experience in patients with chronic liver disease, caution should be exercised in administering EFRIN to patients with liver disease.
Liver Enzymes
In patients with known or suspected history of Hepatitis B or C infection and in patients treated with other medications associated with liver toxicity, monitoring of liver enzymes is recommended. In patients with persistent elevations of serum transaminases to greater than 5 times the upper limit of the normal range, the benefit of continued therapy with EFRIN needs to be weighed against the unknown risks of significant liver toxicity (see section 4.8).
Hepatic side effects
There is some evidence that efavirenz that is associated with three clinical pathological patterns of drug induced liver failure in HIV positive patients of which the sub massive necrosis histological pattern seems to be associated with a high morbidity or mortality risk and may present many months after therapy has been initiated or even stopped. Risk factors include younger age, CD+ counts ≥350 cells/µl and female gender. Patients on EFRIN or efavirenz containing antiretroviral treatment (ART should be regularly monitored for jaundice (including a laboratory bilirubin and liver enzymes) and bleeding tendencies.
EFRIN is not recommended in patients with moderate to severe hepatic impairment (see section 4.3).
Renal insufficiency
The pharmacokinetics of efavirenz have not been studied in patients with renal insufficiency; however, less than 1% of an efavirenz dose is excreted unchanged in the urine, so the impact of renal impairment on efavirenz as contained in EFRIN elimination should be minimal (see section 4.2). There is no experience in patients with severe renal failure and close safety monitoring is recommended in this population.
Elderly patients
Insufficient numbers of elderly patients have been evaluated in clinical studies to determine whether they respond differently than younger patients.
Paediatric population
EFRIN has not been studied in paediatric patients below 3 years of age or who weigh less than 13 kg (see section 4.2).
EFRIN contains lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp-lactase deficiency, or glucose-galactose malabsorption, should not take EFRIN.
4.5. Interaction with other medicinal products and other forms of interaction
EFRIN is an inducer of CYP3A4. Other compounds that are substrates of CYP3A4 may have decreased plasma concentrations when co-administered with EFRIN.
EFRIN exposure may be increased when given with medicines (for example, ritonavir) or food (for example, grapefruit juice), which inhibit CYP3A4 or CYP2B6 activity. Compounds or herbal preparations (for example Ginkgo biloba extracts and St. John’s wort) which induce these enzymes may give rise to decreased plasma concentrations of EFRIN. Concomitant use of St. John’s wort is contraindicated (see section 4.3). Concomitant use of Ginkgo biloba extracts is not recommended (see section 4.4).
QT Prolonging Drugs
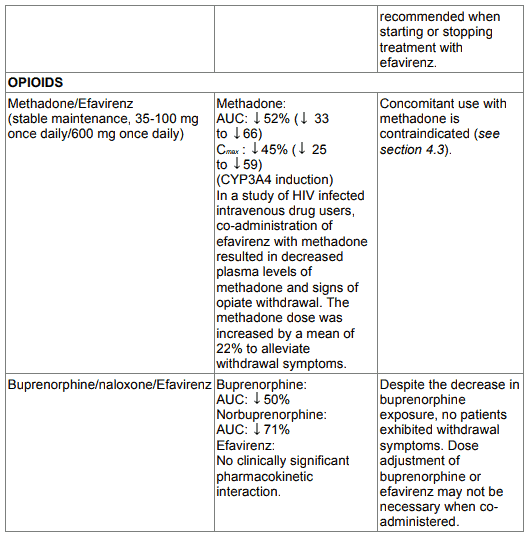
EFRIN is contraindicated with concomitant use of medicines (they may cause prolonged QTc interval and Torsade de Pointes) such as: antidysrhythmic of classes IA and III, neuroleptics and antidepressant medicines, certain antibiotics including some medicines of the following classes: macrolides, fluoroquinolones, imidazole, and triazole antifungal medicines, certain non-sedating antihistaminics (terfenadine), cisapride, flecainide, certain antimalarials and methadone (see section 4.3).
Saquinavir
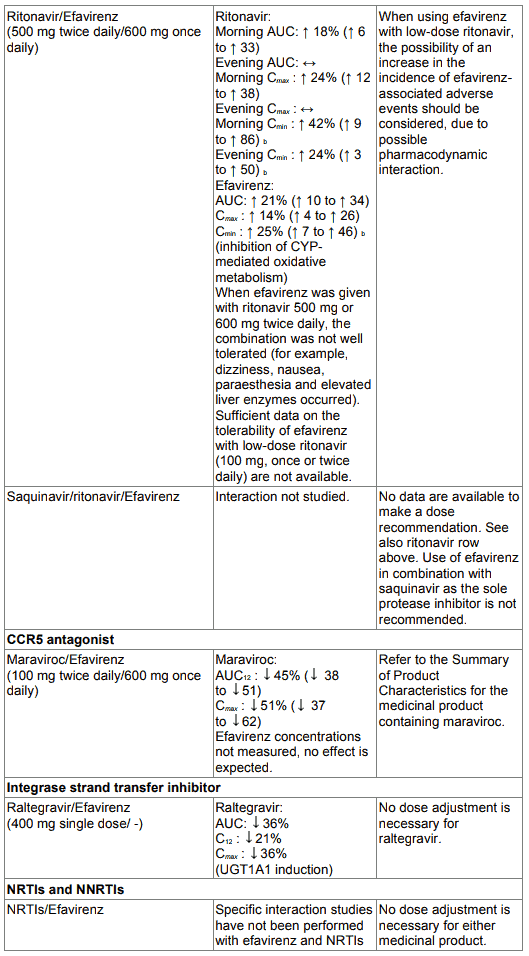
When saquinavir (1,200 mg given 3 times a day, soft capsule formulation) was given with efavirenz the saquinavir AUC and Cmax were decreased by 62 % and 50 % respectively. Use of EFRIN in combination with saquinavir as the sole protease inhibitor is not recommended.
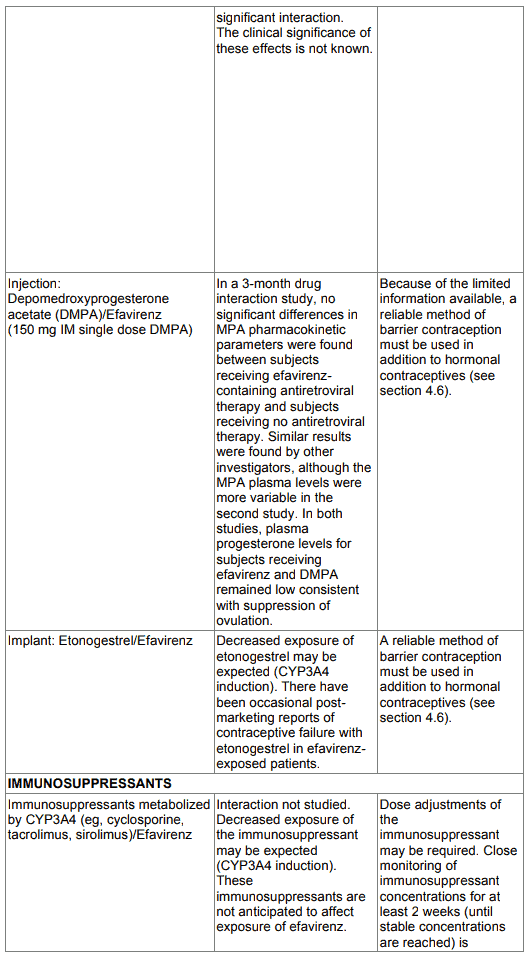
Oral contraceptives
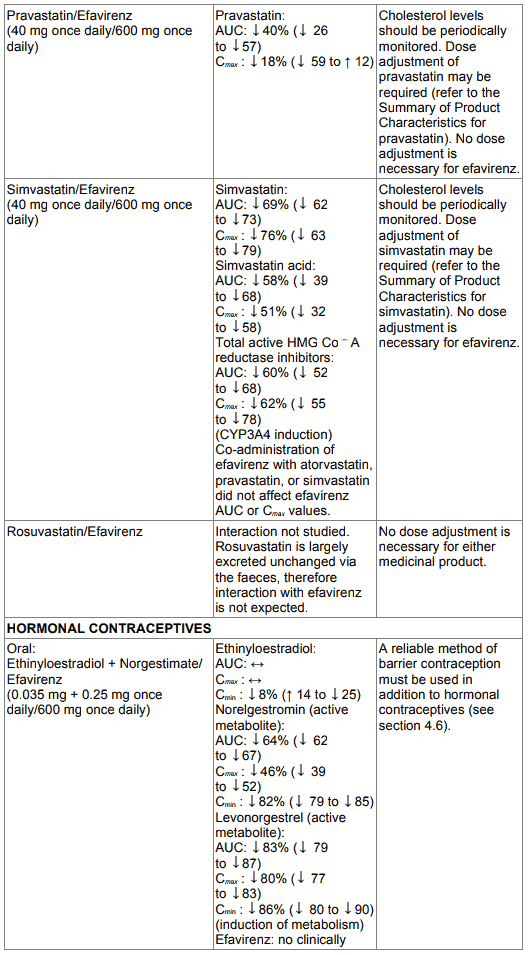
Only the ethinyl oestradiol component of oral contraceptives has been studied. The AUC following a single dose of ethinyl oestradiol was increased (37 %) after multiple dosing of efavirenz. No significant changes were observed in Cmax of ethinyl oestradiol. The clinical significance of these effects is not known. No effect of a single dose of ethinyl oestradiol on efavirenz Cmax or AUC was observed. Because the potential interaction of EFRIN with oral contraceptives has not been fully characterised, a reliable method of barrier contraception must be used in addition to oral contraceptives (see section 4.6).
Contraindications of concomitant use
EFRIN must not be administered concurrently with terfenadine, cisapride, midazolam, triazolam, pimozide, bepridil, or ergot alkaloids (for example, ergotamine, dihydroergotamine, ergonovine, and methylergonovine), since inhibition of their metabolism may lead to serious, life-threatening events (see section 4.3).
Elbasvir/grazoprevir
Concomitant administration of EFRIN with elbasvir/grazoprevir is contraindicated because it may lead to loss of virologic response to elbasvir/grazoprevir. This loss is due to significant decreases in elbasvir and grazoprevir plasma concentrations caused by CYP3A4 induction. (see section 4.3).
St. John’s Wort (Hypericum perforatum)
Patients on EFRIN should not concomitantly use products containing St. John’s Wort (Hypericum perforatum) since it may be expected to result in reduced plasma concentrations of EFRIN. This effect is due to an induction of CYP3A4 and may result in loss of therapeutic effect and development of resistance.
Metamizole
Co-administration of EFRIN with metamizole, which is an inducer of metabolising enzymes including CYP2B6 and CYP3A4 may cause a reduction in plasma concentrations of EFRIN with potential decrease in clinical efficacy. Therefore, caution is advised when metamizole and EFRIN are administered concurrently; clinical response and/or drug levels should be monitored as appropriate.
Cannabinoid Test interaction
Efavirenz does not bind to cannabinoid receptors. False positive urine cannabinoid test results have been reported in uninfected volunteers who received EFRIN. False positive test results have only been observed with the CEDIA DAU Multi-Level THC assay, which is used for screening, and have not been observed with other cannabinoid assays tested including tests used for confirmation of positive results.
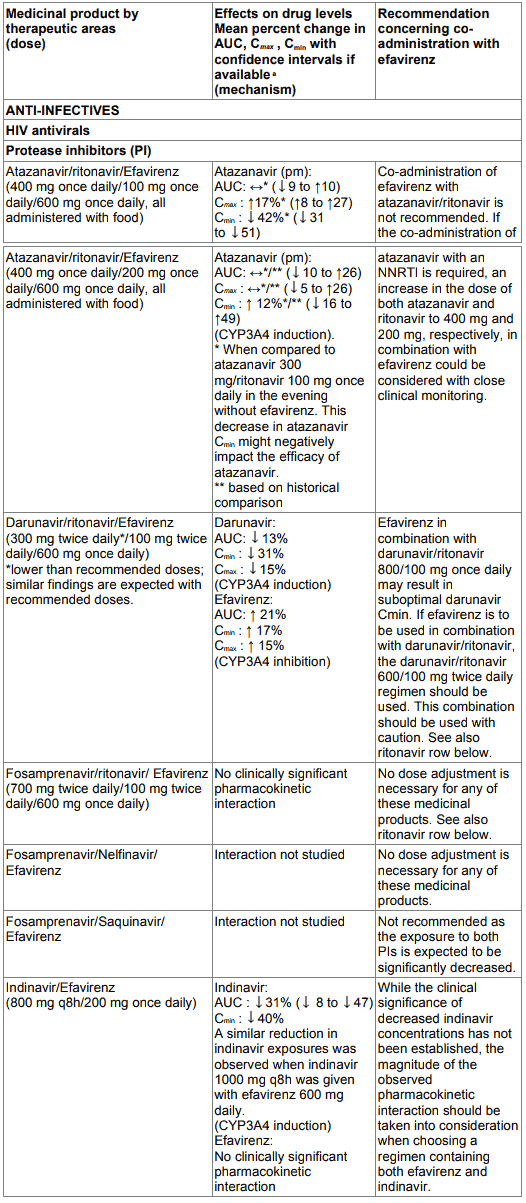
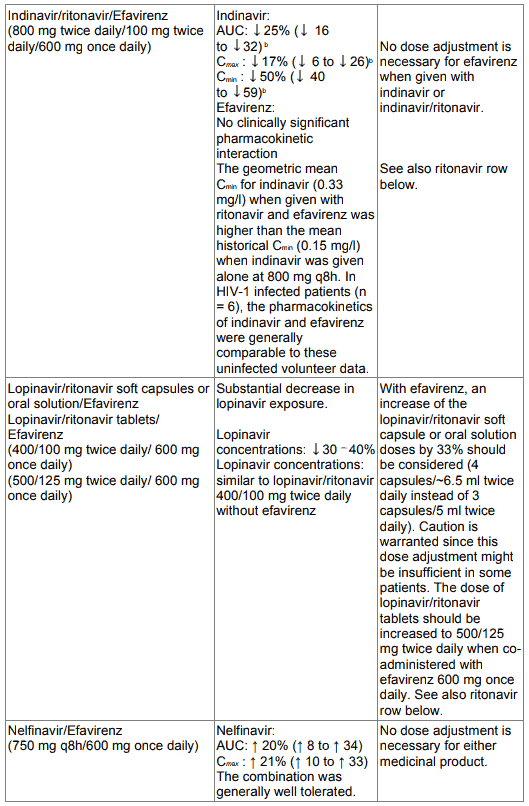
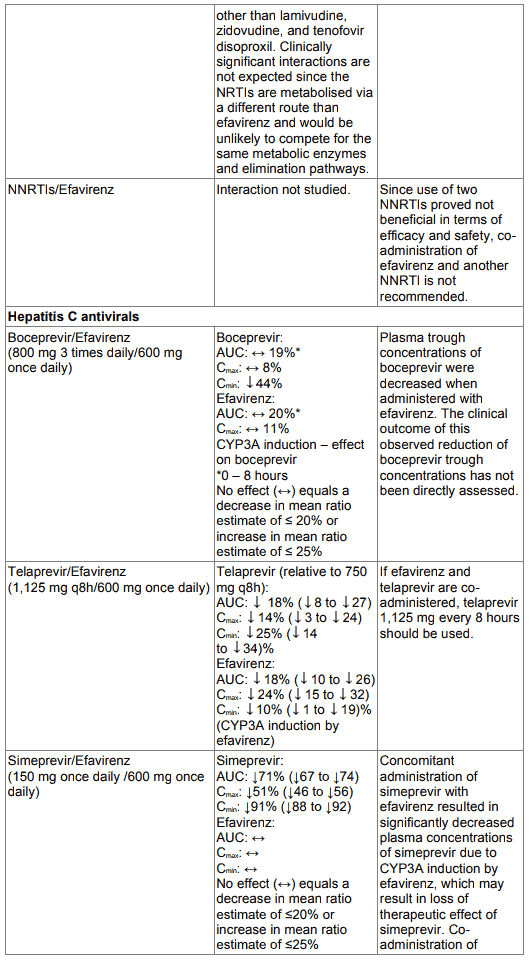
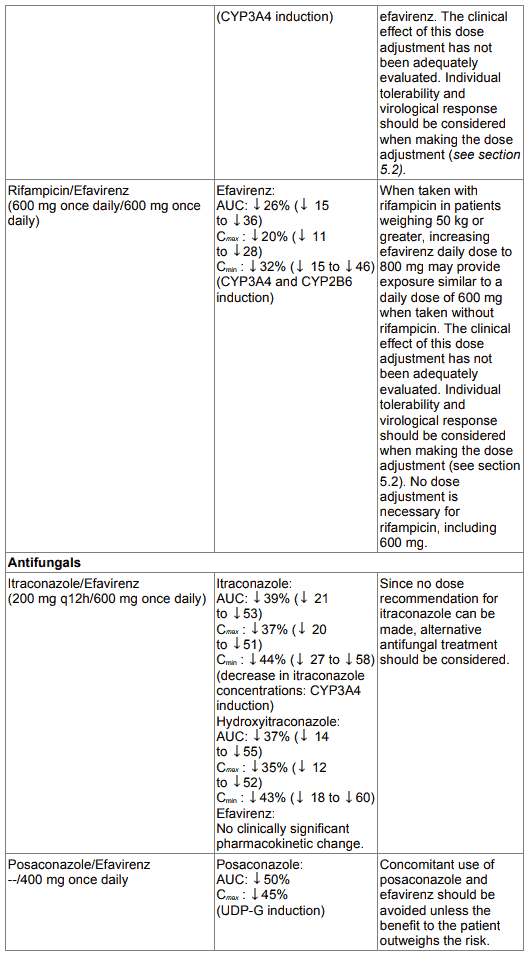
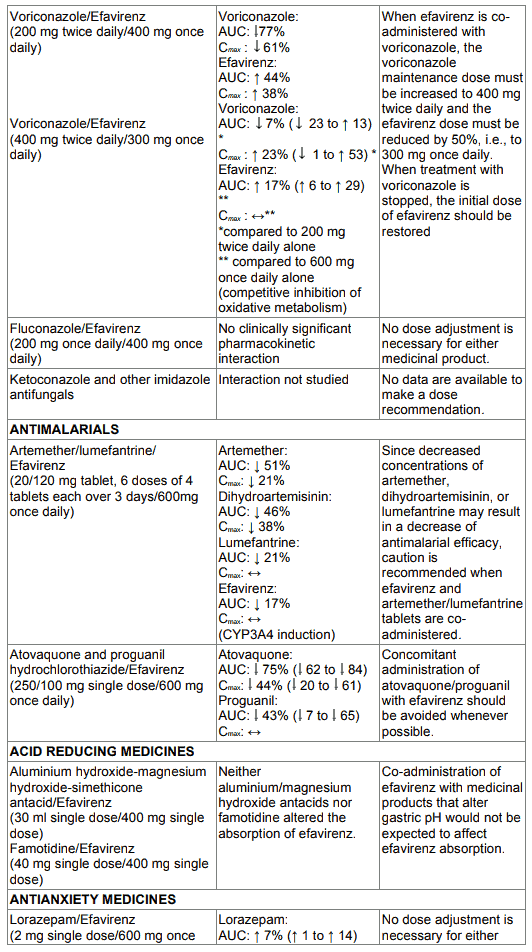
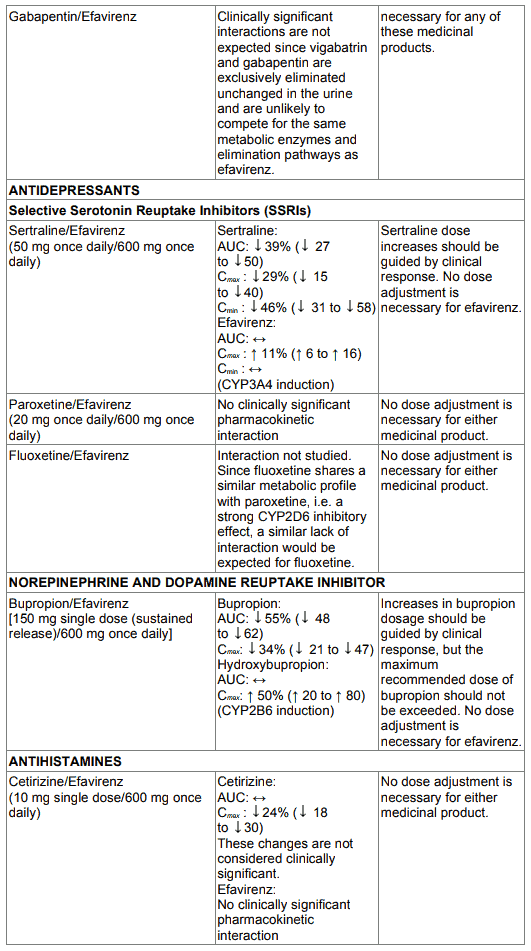
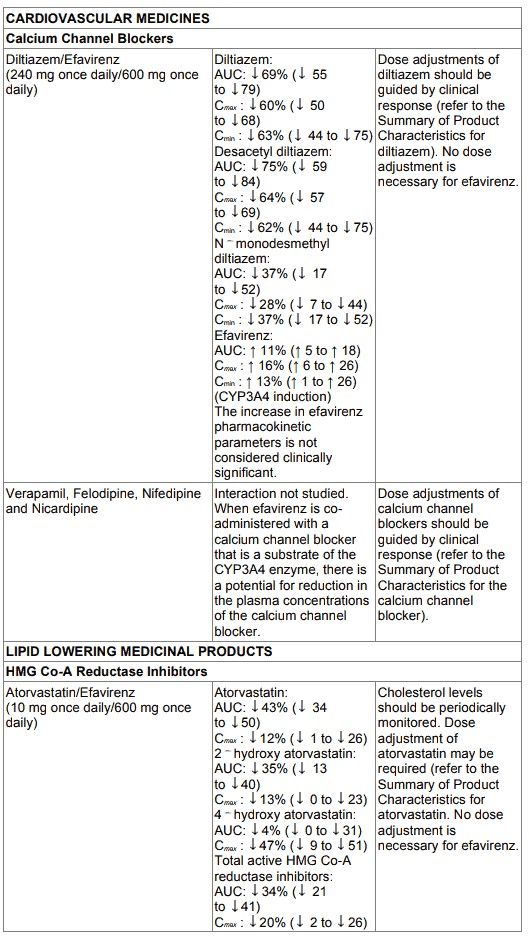
Table 1. Interactions between efavirenz and other medicinal products in adults:
4.6. Pregnancy and lactation
Women of childbearing potential / Contraception in males and females
Women of childbearing potential should undergo pregnancy testing prior to initiation of EFRIN (see section 4.3). Barrier contraception should always be used in combination with other methods of contraception (oral or other hormonal contraceptives e.g. injectable or implant contraception) (see section 4.3). Because of the long half-life of efavirenz, use of adequate contraceptive measures for 12 weeks after discontinuation of efavirenz is recommended.
Pregnancy
The use of EFRIN during pregnancy contraindicated as teratogenicity has been noted. Malformations have been observed in foetuses from efavirenz-treated monkeys that received doses, which resulted in plasma concentrations similar to those in humans given 600 mg/day; therefore pregnancy should be avoided in women receiving EFRIN.
Breastfeeding
The safety in lactation has not been established. Since animal data suggest that the substance may be passed into breast milk, it is recommended that mothers taking EFRIN do not breastfeed their infants.
4.7. Effects on ability to drive and use machines
EFRIN may cause dizziness, impaired concentration, and/or drowsiness. Patients should be instructed that if they experience these symptoms they should avoid potentially hazardous tasks such as driving or operating machinery.
4.8. Undesirable effects
a. Summary of the safety profile
The most frequently reported adverse reactions of EFRIN of at least moderate severity reported were rash, dizziness, nausea, headache and fatigue. The most notable adverse reactions associated with EFRIN are rash and nervous system symptoms. Nervous system symptoms usually begin soon after therapy onset and generally resolve after the first 2-4 weeks. Severe skin reactions such as Stevens-Johnson syndrome and erythema multiforme; psychiatric adverse reactions including severe depression, death by suicide, and psychosis like behaviour; and seizures have been reported in patients treated with EFRIN. The administration of EFRIN with food may increase efavirenz exposure and may lead to an increase in the frequency of adverse reactions (see section 4.4).
Tabulated list of adverse reactions:
| Body System | Undesirable effect | ||
|---|---|---|---|
| Frequent | Less frequent | Frequency not known | |
| Immune system disorders | allergic reaction, erythema multiforme, Stevens-Johnson syndrome immuno-allergic reaction liver injury/failure1 | ||
| Endocrine disorders | pancreatitis | ||
| Metabolism and nutrition disorders | Hypertriglyceridaemia2 | anorexia hypercholesterolaemia2 | increased appetite; redistribution/ accumulation of body fat; weight gain and weight loss |
| Psychiatric disorders | insomnia abnormal dreams, anxiety, depression2 | abnormal thinking; agitation; aggravated depression; amnesia; anxiety; apathy; confusion; delirium; emotional lability; euphoria; hallucinations; psychosis; stupor; mania; suicide attempt; suicide ideation; catatonia2 | neurosis; paranoid reactions; completed suicide2 |
| Nervous system disorders | dizziness; fatigue; headache; impaired concentration; somnolence | ataxia; convulsions; impaired co- ordination; malaise; neuralgia; peripheral neuropathy | abnormal co- ordination; hypoaesthesia; neuropathy and pain; paraesthesia; speech disorder; tremor |
| Eye disorders | abnormal vision | ||
| Ear and labyrinth disorders | tinnitus, vertigo | ||
| Cardiac disorders | palpitations and tachycardia | ||
| Vascular disorders | flushing | hot flushes | |
| Respiratory, thoracic and mediastinal disorders | asthma | dyspnoea; sinusitis; upper respiratory tract infections | |
| Gastrointestinal disorders | nausea; vomiting; taste perversion | abdominal pain and gastroesophageal reflux | constipation; diarrhoea; dyspepsia; gastritis; gastroenteritis; malabsorption pancreatitis2 |
| Hepato-biliary disorders | aspartate amino transferase (AST) increased, alanine aminotransferase (ALT) increased, gammaglutamyltran sferase (GGT) increased | hepatitis and hepatic enzyme increase | hepatic failure |
| Skin and subcutaneous tissue disorders | pruritus; rash | alopecia; eczema; folliculitis; skin exfoliation and urticarial; Stevens- Johnson syndrome2 | acne; increased sweating; nail disorders; seborrhoea; skin discolouration |
| Musculoskeletal, connective tissue and bone disorders | arthralgia and myalgia | Myopathy, osteonecrosis | |
| Reproductive system and breast disorders | gynaecomastia2 | decreased libido; increased libido; impotence | |
| Congenital and familial/genetic disorders | |||
| General disorders and administrative site conditions | fatigue | asthenia | alcohol intolerance; influenza-like symptoms |
Description of selected adverse reactions
Rashes are usually mild to moderate maculopapular skin eruptions that occur within the first two weeks of initiating therapy with EFRIN. In most patients rash resolves with continuing therapy with EFRIN within one month. EFRIN can be reinitiated in patients interrupting therapy because of rash. Use of appropriate antihistamines and/or corticosteroids is recommended when EFRIN is restarted.
Experience with EFRIN in patients who discontinued other antiretroviral medicines of the NNRTI class is limited. Reported rates of recurrent rash following a switch from nevirapine to efavirenz therapy, primarily based on retrospective cohort data from published literature, range from 13 to 18%.
Psychiatric symptoms
Serious psychiatric adverse reactions have been reported in patients treated with EFRIN.
Specific serious psychiatric events:
- severe depression
- suicidal ideation
- non-fatal suicide attempts
- aggressive behaviour
- paranoid reactions
- manic reactions
Patients with a history of psychiatric disorders appear to be at greater risk of these serious psychiatric adverse reactions. There have also been post-marketing reports of death by suicide, delusions, psychosis-like behaviour and catatonia.
Nervous system symptoms
In clinical controlled trials, frequently reported adverse reactions included, but were not limited to dizziness, insomnia, somnolence, impaired concentration and abnormal dreaming. Nervous system symptoms usually begin during the first one or two days of therapy and generally resolve after the first 2-4 weeks. In a study of uninfected volunteers, a representative nervous system symptom had a median time to onset of 1 hour post-dose and a median duration of 3 hours. Nervous system symptoms may occur more frequently when efavirenz is taken concomitantly with meals possibly due to increased efavirenz plasma levels (see section 5.2).
Dosing at bedtime seems to improve the tolerability of these symptoms and can be recommended during the first weeks of therapy and in patients who continue to experience these symptoms (see section 4.2). Dose reduction or splitting the daily dose has not been shown to provide benefit.
Laboratory abnormalities
Liver enzymes
Raised liver enzyme values have occurred, particularly in patients with viral hepatitis. Raised serum-cholesterol and triglyceride concentrations have been reported.
Elevations of AST and ALT were seen in patients treated with 600 mg of EFRIN. Elevations of GGT to greater than 5 times the upper limit of the normal range were observed in patients treated with 600 mg EFRIN and in a greater percentage in patients seropositive for Hepatitis B or C.
Lipids
An increase in total cholesterol of 10 to 20% has been observed in uninfected volunteers receiving efavirenz. Increases in non-fasting total cholesterol and HDL of approximately 20% and 25% respectively were observed in patients treated with efavirenz+SDV+3TC, and of approximately 40% and 35% in patients treated with efavirenz+IDV. The effects of efavirenz on triglycerides and LDL were not well-characterised. The clinical significance of these findings is unknown (see section 4.4).
Metabolic parameters
Weight and levels of blood lipids and glucose may increase during antiretroviral therapy (see section 4.4).
Paediatric population
Undesirable effects in children were generally similar to those of adult patients. Rash was reported more frequently in children treated with EFRIN and was more often of higher grade than in adults. Prophylaxis with appropriate antihistamines prior to initiating therapy with EFRIN in children may be considered.
Other special populations
Liver enzymes in hepatitis B or C co-infected patients
Patients treated with efavirenz-containing regimens, as contained in EFRIN, were seropositive at screening for hepatitis B (surface antigen positive) and/or C (hepatitis C antibody positive).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicine is important. It allows continued monitoring of the benefit/risk balance of the medicine. Healthcare professionals are asked to report any suspected adverse reactions to SAHPRA via the “Report Drug Reaction Process”, found online under SAHPRA’s safety publications: https://www.sahpra.org.za/.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.