ELLA Tablet Ref.[10802] Active ingredients: Ulipristal
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
When taken immediately before ovulation is to occur, ella postpones follicular rupture. The likely primary mechanism of action of ulipristal acetate for emergency contraception is therefore inhibition or delay of ovulation; however, alterations to the endometrium that may affect implantation may also contribute to efficacy.
12.2. Pharmacodynamics
Ulipristal acetate is a selective progesterone receptor modulator with antagonistic and partial agonistic effects (a progesterone agonist/antagonist) at the progesterone receptor. It binds the human progesterone receptor and prevents progesterone from occupying its receptor.
The pharmacodynamics of ulipristal acetate depends on the timing of administration in the menstrual cycle. Administration in the mid-follicular phase causes inhibition of folliculogenesis and reduction of estradiol concentration.
Pharmacodynamic data showed that administration of ella to 34 women in the late follicular phase postponed follicular rupture for at least 5 days in all (100%) of 8 subjects who took ella before the luteinizing hormone (LH) surge and 11 (79%) of 14 subjects who took ella immediately before ovulation (when LH has already started to rise). However, treatment was not effective in postponing follicular rupture when administered on the day of LH peak.
Dosing in the early luteal phase does not significantly delay endometrial maturation but decreases endometrial thickness by 0.6 ± 2.2 mm (mean ± SD).
Hormonal Contraceptives after ella intake
When a combined oral contraceptive pill (COC) containing ethinyl estradiol 30 µg + levonorgestrel 150 µg was started the day after ella intake during the follicular phase, ella did not interfere with the COC's ability to suppress ovarian activity, as assessed by measurement of follicle size via transvaginal ultrasound, combined with serum progesterone and estradiol levels: ovarian activity was suppressed in 61.5% (24/39) of subjects receiving ella plus COC and 62.2% (23/37) of subjects receiving a placebo plus the COC. The incidence of ovulation was similar between the group who received ella plus the COC [33.3% (13/39)] and the group who received a placebo plus the COC [32.4% (12/37)] [see Warnings and Precautions (5.5) and Drug Interactions (7.3)].
The initiation of a desogestrel 75 µg "progestin-only pill" the day after ella intake during the follicular phase was associated with a higher incidence of ovulation in the six days following ella intake compared to an ella-only treatment group, and a relatively slower onset (3 to 4 days) of thickened cervical mucus compared to a group given desogestrel without prior ella intake (2 days), suggesting an effect of prior use of ella on the ability of desogestrel to inhibit mucus permeability [See Warnings and Precautions (5.5) and Drug Interactions (7.1; 7.3)].
12.3. Pharmacokinetics
Absorption
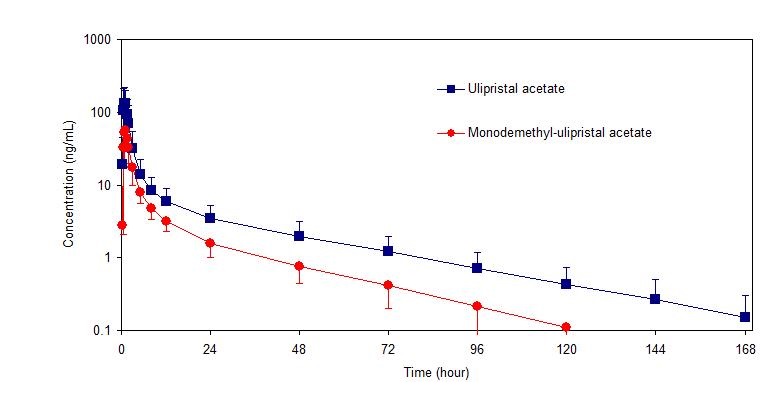
Following a single dose administration of ella in 20 women under fasting conditions, maximum plasma concentrations of ulipristal acetate and the active metabolite, monodemethyl-ulipristal acetate, were 176 and 69 ng/ml and were reached at 0.9 and 1 hour, respectively.
Figure 1. Mean (± SD) Plasma Concentration-time Profile of Ulipristal Acetate and Monodemethyl-ulipristal Acetate Following Single Dose Administration of 30 mg Ulipristal Acetate:
Table 2. Pharmacokinetic Parameter Values Following Administration of ella (ulipristal acetate) Tablet 30 mg to 20 Healthy Female Volunteers under Fasting Conditions:
| Mean (± SD) | |||||
|---|---|---|---|---|---|
| Cmax (ng/ml) | AUC0-t (ng•hr/ml) | AUC0-∞ (ng•hr/ml) | tmax (hr)* | t1/2 (hr) | |
| Ulipristal acetate | 176 (89) | 548 (259) | 556 (260) | 0.9 (0.5-2.0) | 32 (6.3) |
| Monodemethyl- ulipristal acetate | 69 (26) | 240 (59) | 246 (59) | 0.9 (0.8-2.0) | 27 (6.9) |
Cmax = maximum concentration
AUC0-t = area under the drug concentration curve from time 0 to time of last determinable concentration
AUC0-∞ = area under the drug concentration curve from time 0 to infinity
tmax = time to maximum concentration
t1/2 = elimination half-life
* Median (range)
Effect of food: Administration of ella together with a high-fat breakfast resulted in approximately 40-45% lower mean Cmax, a delayed tmax (from a median of 0.75 hours to 3 hours) and 20-25% higher mean AUC0-∞ of ulipristal acetate and monodemethyl-ulipristal acetate compared with administration in the fasting state. These differences are not expected to impair the efficacy or safety of ella to a clinically significant extent; therefore, ella can be taken with or without food.
Distribution
Ulipristal acetate is highly bound (>94%) to plasma proteins, including high density lipoprotein, alpha-l-acid glycoprotein, and albumin.
Metabolism
Ulipristal acetate is metabolized to mono-demethylated and di-demethylated metabolites. In vitro data indicate that this is predominantly mediated by CYP3A4. The mono-demethylated metabolite is pharmacologically active.
Excretion
The terminal half-life of ulipristal acetate in plasma following a single 30 mg dose is estimated to 32.4 ± 6.3 hours.
Drug interactions
CYP3A4 inducers
When a single 30 mg dose of ulipristal acetate was administered following administration of the strong CYP3A4 inducer, rifampin 600 mg once daily for 9 days, Cmax and AUC of ulipristal acetate decreased by 90% and 93% respectively. The Cmax and AUC of monodemethyl-ulipristal acetate decreased by 84% and 90% respectively [see Drug Interactions (7.1)].
CYP3A4 inhibitors
When a single 10 mg dose of ulipristal acetate was administered following administration of the strong CYP3A4 inhibitor, ketoconazole 400 mg once daily for 7 days, Cmax and AUC of ulipristal acetate increased by 2- and 5.9-fold, respectively. While the AUC of monodemethyl-ulipristal acetate increased by 2.4-fold, Cmax of monodemethyl-ulipristal acetate decreased by 47%. There was no in vivo drug-drug interaction study between ulipristal acetate 30 mg and CYP3A4 inhibitors [see Drug Interactions (7.1)].
In vitro studies demonstrated that ella does not induce or inhibit the activity of cytochrome P450 enzymes.
P-glycoprotein (P-gp) transporter
In vitro data indicate that ulipristal may be an inhibitor of P-gp at clinically relevant concentrations. When a single 60 mg dose of fexofenadine, a substrate of P-gp glycoprotein, was administered 1.5 hours after the administration of a single 10 mg dose of ulipristal acetate, there was no increase in Cmax or AUC of fexofenadine.
Breast Cancer Resistance Protein (BCRP) transporter
In vitro data indicate that ulipristal acetate may be an inhibitor of BCRP at the intestinal level.
The effects of ella on P-gp and BCRP transporters are unlikely to have any clinical consequences when considering ella's single dose treatment regimen, although there was no in vivo drug interaction study between ulipristal acetate 30 mg (ella) and substrates of P-pg and BCRP transporters.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Carcinogenicity potential was evaluated in rats and mice.
Sprague Dawley rats were exposed to ulipristal acetate daily for 99-100 weeks at doses of 1, 3, or 10 mg/kg/day, representing exposures up to 31 times higher than exposures at the maximum recommended human dose (MRHD). There were no drug-related neoplasms in male rats. In female rats, potential treatment-related neoplastic findings were limited to adrenal cortical adenomas in the intermediate dose group (3 mg/kg/day). Despite the increase, this incidence of adrenal cortical adenomas in females may not be relevant to clinical use.
Tg.rasH2 transgenic mice were exposed to ulipristal acetate for 26 weeks at doses of 5, 45, or 130 mg/kg/day, representing exposures 100 times higher than exposures at the MRHD. There was no drug-related increase in neoplasm incidence in male or female mice.
Genotoxicity
Ulipristal acetate was not genotoxic in the Ames assay, in vitro mammalian assays utilizing mouse lymphoma cells and human peripheral blood lymphocytes, and in an in vivo micronucleus assay in mice.
Impairment of Fertility
Single oral doses of ulipristal acetate prevented ovulation in 50% of rats at 2 times the human exposure based on body surface area (mg/m²). Single doses of ulipristal acetate given on post-coital days 4 or 5 prevented pregnancy in 80-100% of rats and in 50% of rabbits when given on post-coital days 5 or 6 at drug exposures 4 and 12 times the human exposure based on body surface area. Lower doses administered for 4 days to rats and rabbits were also effective at preventing ovulation and pregnancy.
14. Clinical Studies
Two multicenter clinical studies evaluated the efficacy and safety of ella. An open-label study provided the primary data to support the efficacy and safety of ulipristal acetate for emergency contraception when taken 48 to 120 hours after unprotected intercourse. A single-blind comparative study provided the primary data to support the efficacy and safety of ulipristal acetate for emergency contraception when taken 0 to 72 hours after unprotected intercourse and provided supportive data for ulipristal acetate for emergency contraception when taken >72 to 120 hours after unprotected intercourse. Women in both studies were required to have a negative pregnancy test prior to receiving emergency contraception. The primary efficacy analyses were performed on subjects less than 36 years of age who had a known pregnancy status after taking study medication.
Table 3. Summary of Clinical Trial Results for Women Who Received a Single Dose of ella (30 mg Ulipristal Acetate):
| Open-Label Study 48 to 120 Hours* | Single-Blind Comparative Study 0 to 72 Hours* | |
|---|---|---|
| N=1,242 | N=844 | |
| Expected Pregnancy Rate** | 5.5 | 5.6 |
| Observed Pregnancy Rate** (95% confidence interval) | 2.2 (1.5, 3.2) | 1.9 (1.1, 3.1) |
* Time after unprotected intercourse when ella was taken
** Number of pregnancies per 100 women at risk for pregnancy
14.1 Open-Label Study
This study was a multicenter open-label trial conducted at 40 family planning clinics in the United States. Healthy women with a mean age of 24 years who requested emergency contraception 48 to 120 hours after unprotected intercourse received a dose of 30 mg ulipristal acetate (ella). The median BMI for the study subjects was 25.3 and ranged from 16.1 to 61.3 kg/m².
Twenty-seven pregnancies occurred in 1,242 women aged 18 to 35 years evaluated for efficacy. The number of pregnancies expected without emergency contraception was calculated based on the timing of intercourse with regard to each woman's menstrual cycle. ella statistically significantly reduced the pregnancy rate, from an expected rate of 5.5% to an observed rate of 2.2%, when taken 48 to 120 hours after unprotected intercourse.
14.2 Single-Blind Comparative Study
This study was a multicenter, single-blind, randomized comparison of the efficacy and safety of 30 mg ulipristal acetate (ella) to levonorgestrel (another form of emergency contraception). Subjects were enrolled at 35 sites in the U.S., the United Kingdom and Ireland, with the majority (66%) having been enrolled in the U.S. Healthy women with a mean age of 25 years who requested emergency contraception within 120 hours of unprotected intercourse were enrolled and randomly allocated to receive ella or levonorgestrel 1.5 mg. The median BMI for the study subjects was 25.3 and ranged from 14.9 to 70.0 kg/m².
In the ella group, 16 pregnancies occurred in 844 women aged 16 to 35 years when emergency contraception was taken 0 to 72 hours after unprotected intercourse. The number of pregnancies expected without emergency contraception was calculated based on the timing of intercourse with regard to each woman's menstrual cycle; ella statistically significantly reduced the pregnancy rate, from an expected 5.6% to an observed 1.9%, when taken within 72 hours after unprotected intercourse. There were no pregnancies observed in the women who were administered ella more than 72 hours after unprotected intercourse (10% of women who received ella).
14.3 Pooled Analysis
Data from the two studies were pooled to provide a total efficacy population of women treated with ulipristal acetate up to 120 hours after UPI. Time Trend analysis for the five 24-hour intervals from 0 to 120 hours between unprotected intercourse and treatment was conducted. There were no significant differences in the observed pregnancy rates across the five time intervals.
Subgroup analysis of the pooled data by BMI showed that for women with BMI >30 kg/m² (16% of all subjects), the observed pregnancy rate was 3.1% (95% CI: 1.7, 5.7), which was not significantly reduced compared to the expected pregnancy rate of 4.5% in the absence of emergency contraception taken within 120 hours after unprotected intercourse. In the comparative study, a similar effect was seen for the comparator emergency contraception drug, levonorgestrel 1.5 mg. For levonorgestrel, when used by women with BMI >30 kg/m², the observed pregnancy rate was 7.4% (95% CI: 3.9, 13.4), compared to the expected pregnancy rate of 4.4% in the absence of emergency contraception taken within 72 hours after unprotected intercourse.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.