EMGALITY Solution for injection Ref.[7656] Active ingredients: Galcanezumab
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Eli Lilly Nederland B.V., Papendorpseweg 83, 3528BJ, Utrecht, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: analgesics, calcitonin gene-related peptide (CGRP) antagonists
ATC code: N02CD02
Mechanism of action
Galcanezumab is a humanised IgG4 monoclonal antibody that binds calcitonin gene-related peptide (CGRP) thus preventing its biological activity. Elevated blood concentrations of CGRP have been associated with migraine attacks. Galcanezumab binds to CGRP with high affinity (KD = 31 pM) and high specificity (>10,000-fold vs related peptides adrenomedullin, amylin, calcitonin and intermedin).
Clinical efficacy and safety
The efficacy and safety of galcanezumab has been studied in 3 phase 3, randomized, placebocontrolled, double-blind studies in adult patients (N=2886). The 2 episodic migraine studies (EVOLVE-1 and EVOLVE-2) enrolled patients who met International Classification of Headache Disorders (ICHD) criteria for a diagnosis of migraine with or without aura with 4-14 migraine headache days per month. The chronic migraine study (REGAIN) enrolled patients who met ICHD criteria for chronic migraine with ≥15 headache days per month, of which at least 8 had the features of migraine. Patients with recent acute cardiovascular events (including MI, unstable angina, CABG, stroke, DVT) and/or those deemed to be at serious cardiovascular risk were excluded from the galcanezumab clinical trials. Patients >65 years of age were also excluded.
Patients received placebo, galcanezumab 120 mg/month (with an initial loading dose of 240 mg for the first month) or galcanezumab 240 mg/month and were allowed to use medication for the acute treatment of migraine. Across the 3 studies, patients were predominantly female (>83%) with a mean age of 41 years, and an average migraine history of 20 to 21 years. Approximately one-third of patients across the studies had at least 1 prior failure on a migraine prophylactic treatment for efficacy reasons and approximately 16% of patients across the studies had at least 2 prior failure on a prophylactic treatment for efficacy reasons.
In all 3 studies, the overall mean change from baseline in number of monthly Migraine Headache Days (MHDs) was the primary efficacy measure. Response rate is the mean percentage of patients meeting a defined threshold in the reduction of the number of monthly MHDs (≥50%, ≥75% and 100%) across the double-blind treatment period. The impact of migraine on functioning was assessed by the Role Function-Restrictive domain of the Migraine-Specific Quality of Life Questionnaire (MSQ) version 2.1, and by the Migraine Disability Assessment (MIDAS) Questionnaire. The MSQ measures impact of migraine on work or daily activities, relationships with family and friends, leisure time, productivity, concentration, energy, and tiredness. Scoring ranges from 0 to 100, with higher scores indicating less impairment, that is, patients experience fewer restrictions on the performance of day-to-day activities. For the MIDAS, higher scores indicate more disability. The baseline scores of the MIDAS reflected severe migraine related disability of patients in EVOLVE-1 and EVOLVE-2 (mean of 33.1) and a very severely disabled population (mean of 67.2) in REGAIN.
Episodic migraine
Studies EVOLVE-1 and EVOLVE-2 had a 6 month, double-blind, placebo-controlled treatment period. Completion rate of the double-blind treatment phase for patients who received galcanezumab ranged from 82.8% to 87.7%.
Both galcanezumab 120 mg and 240 mg treatment groups demonstrated statistically significant and clinically meaningful improvements from baseline compared to placebo on mean change in MHD (see Table 2). Patients treated with galcanezumab had greater response rates and greater reductions in the number of monthly MHDs that acute medication was taken compared with placebo-treated patients. Galcanezumab-treated patients had a greater improvement in functioning (as measured by the MSQ Role Function-Restrictive domain score) compared with placebo-treated patients, beginning at month 1. More patients treated with galcanezumab achieved clinically significant levels of improvement in functioning (responder rate based on MSQ Role Function Restrictive domain) compared with those treated with placebo. Galcanezumab was associated with a statistically significant reduction in disability over placebo.
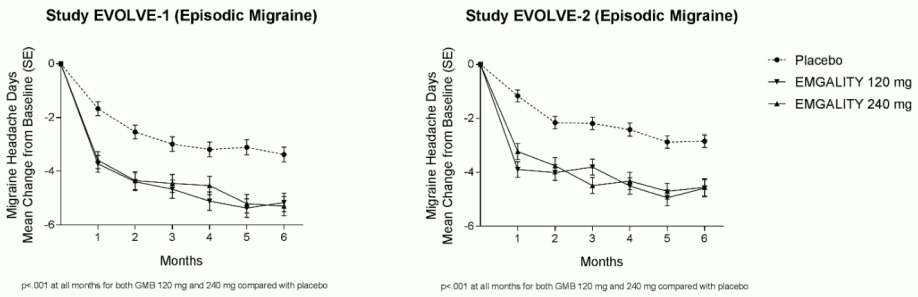
Compared with placebo-treated patients, patients treated with galcanezumab 120 mg or 240 mg had significantly greater mean decreases from baseline in the number of monthly MHDs at month 1 and at all subsequent months up to month 6 (see Figure 1). Additionally, in month 1, patients treated with galcanezumab (loading dose of 240 mg) demonstrated significantly fewer weekly MHDs compared with placebo-treated patients, at week 1 and each subsequent week.
Figure 1. Reduction in monthly migraine headache days over time in studies EVOLVE-1 and EVOLVE-2:
Table 2. Efficacy and patient reported outcome measures:
| EVOLVE 1 – Episodic Migraine | EVOLVE 2 – Episodic Migraine | |||||
|---|---|---|---|---|---|---|
| Emgality | Placebo | Emgality | Placebo | |||
| 120 mg | 240 mg | 120 mg | 240 mg | |||
| N=210 | N=208 | N=425 | N=226 | N=220 | N=450 | |
| Efficacy Outcomesa | ||||||
| MHD | ||||||
| Baseline | 9.21 | 9.14 | 9.08 | 9.07 | 9.06 | 9.19 |
| Mean Change | -4.73 | -4.57 | -2.81 | -4.29 | -4.18 | -2.28 |
| Treatment Difference | -1.92 | -1.76 | -2.02 | -1.90 | ||
| CI95% | (-2.48, -1.37) | (-2.31, -1.20) | (-2.55, -1.48) | (-2.44, -1.36) | ||
| P-value | <.001d | <.001d | <.001d | <.001d | ||
| 50% MHD Responders | ||||||
| Percentage, % | 62.3 | 60.9 | 38.6 | 59.3 | 56.5 | 36.0 |
| P-value | <.001d | <.001d | <.001d | <.001d | ||
| ≥75% MHD Responders | ||||||
| Percentage, % | 38.8 | 38.5 | 19.3 | 33.5 | 34.3 | 17.8 |
| P-value | <.001d | <.001d | <.001d | <.001d | ||
| 100% MHD Responders | ||||||
| Percentage, % | 15.6 | 14.6 | 6.2 | 11.5 | 13.8 | 5.7 |
| P-value | <.001d | <.001d | <.001d | <.001d | ||
| MHD with Acute Medication Use | ||||||
| Baseline | 7.42 | 7.34 | 7.38 | 7.47 | 7.47 | 7.62 |
| Mean Change | -3.96 | -3.76 | -2.15 | -3.67 | -3.63 | -1.85 |
| Treatment Difference | -1.81 | -1.61 | -1.82 | -1.78 | ||
| CI95% | (-2.28, -1.33) | (-2.09, -1.14) | (-2.29, -1.36) | (-2.25, -1.31) | ||
| P-value | <.001d | <.001d | <.001d | <.001d | ||
| Patient-reported Outcome Measures | ||||||
| MSQ Role Function-Restrictive Domainb | ||||||
| N | 189 | 184 | 377 | 213 | 210 | 396 |
| Baseline | 51.39 | 48.76 | 52.92 | 52.47 | 51.71 | 51.35 |
| Mean Change | 32.43 | 32.09 | 24.69 | 28.47 | 27.04 | 19.65 |
| Treatment Difference | 7.74 | 7.40 | 8.82 | 7.39 | ||
| CI95% | (5.20, 10.28) | (4.83, 9.97) | (6.33, 11.31) | (4.88, 9.90) | ||
| P-value | <.001d | <.001d | <.001d | <.001d | ||
| MSQ Role Function Restrictive Domain Respondersc | ||||||
| N | 189 | 184 | 377 | 213 | 210 | 396 |
| Percentage, % | 63.5 | 69.6 | 47.2 | 58.2 | 60.0 | 43.4 |
| P-value | <.001f | <.001f | <.001f | <.001f | ||
| MIDAS Total Scoree | ||||||
| N | 177 | 170 | 345 | 202 | 194 | 374 |
| Baseline | 32.93 | 36.09 | 31.84 | 30.87 | 32.75 | 34.25 |
| Mean Change | -21.16 | -20.06 | -14.87 | -21.17 | -20.24 | -12.02 |
| Treatment Difference | -6.29 | -5.19 | -9.15 | -8.22 | ||
| CI95% | (-9.45, -3.13) | (-8.39, -1.98) | (-12.61, -5.69) | (-11.71, -4.72) | ||
| P-value | <.001f | .002f | <.001f | <.001f | ||
N = number of patients; CI95% = 95% confidence interval.
a Efficacy outcomes were evaluated across Months 1-6.
b Evaluated across Months 4-6.
c Defined as those with an improvement of ≥25 points for Episodic Migraine at Months 4-6 average.
d Statistically significant after adjustment for multiple comparisons.
e Evaluated at Month 6.
f Not adjusted for multiple comparisons.
In pooled data from studies EVOLVE-1 and EVOLVE-2, in patients who failed one or more prophylactic treatments for efficacy reasons, the treatment difference for the reduction of mean monthly MHDs observed between galcanezumab 120 mg and placebo was -2.69 days (p<0.001) and between galcanezumab 240 mg and placebo -2.78 days (p<0.001). In patients failing two or more prophylactic treatments, the treatment difference was -2.64 days (p<0.001) between 120 mg and placebo and -3.04 days (p<0.001) between 240 mg and placebo.
Chronic Migraine
Study REGAIN had a 3 month, double-blind, placebo-controlled treatment period followed by a 9 month open-label extension. Approximately 15% of the patients continued concurrent treatment with topiramate or propranolol as allowed by the protocol for prophylaxis of migraine. Completion rate of the double-blind treatment phase for patients who received galcanezumab was 95.3%.
Both galcanezumab 120 mg and 240 mg treatment groups demonstrated statistically significant and clinically meaningful improvements from baseline compared to placebo on mean change in MHD (see Table 3). Patients treated with galcanezumab had greater response rates and greater reductions in the number of monthly MHDs that acute medication was taken compared with placebo-treated patients. Galcanezumab-treated patients had a greater improvement in functioning (as measured by the MSQ Role Function-Restrictive domain score) compared with placebo-treated patients, beginning at month 1. More patients treated with galcanezumab achieved clinically significant levels of improvement in functioning (responder rate based on MSQ Role Function Restrictive domain) compared with those treated with placebo. The 120 mg dose was associated with a statistically significant reduction in disability over placebo.
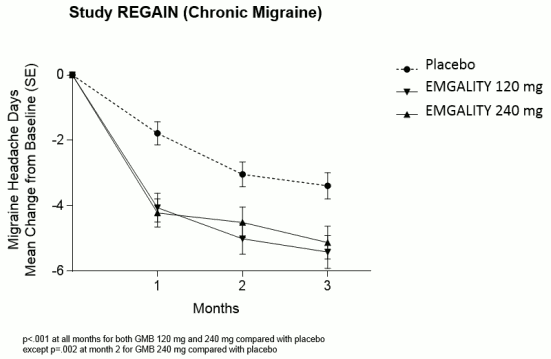
Compared with placebo-treated patients, patients treated with galcanezumab 120 mg or 240 mg had significantly greater mean decreases from baseline in the number of monthly MHDs at the first month and at all subsequent months up to month 3 (see Figure 2). Additionally, in month 1, patients treated with galcanezumab (loading dose of 240 mg) demonstrated significantly fewer weekly MHDs compared with placebo-treated patients, at week 1 and each subsequent week.
Figure 2. Reduction in monthly migraine headache days over time in study REGAIN:
Table 3. Efficacy and patient reported outcome measures:
| REGAIN – Chronic Migraine | |||
|---|---|---|---|
| Emgality | Placebo | ||
| 120mg | 240mg | ||
| N=273 | N=274 | N=538 | |
| Efficacy Outcomesa | |||
| MHD | |||
| Baseline | 19.36 | 19.17 | 19.55 |
| Mean Change | -4.83 | -4.62 | -2.74 |
| Treatment Difference | -2.09 | -1.88 | |
| CI95% | (-2.92, -1.26) | (-2.71, -1.05) | |
| P-value | <.001c | <.001c | |
| ≥50% MHD Responders | |||
| Percentage, % | 27.6 | 27.5 | 15.4 |
| P-value | <.001c | <.001c | |
| ≥75% MHD Responders | |||
| Percentage, % | 7.0 | 8.8 | 4.5 |
| P-value | .031d | <.001c | |
| 100% MHD Responders | |||
| Percentage, % | 0.7 | 1.3 | 0.5 |
| P-value | >.05d | >.05d | |
| MHD with Acute Medication Use | |||
| Baseline | 15.12 | 14.49 | 15.51 |
| Mean Change | -4,74 | -4.25 | -2.23 |
| Treatment Difference | -2.51 | -2.01 | |
| CI95% | (-3.27, -1.76) | (-2,77, -1.26) | |
| P-value | <.001d | <.001c | |
| Patient-reported Outcome Measuresb | |||
| MSQ Role Function-Restrictive Domain | |||
| N | 252 | 253 | 494 |
| Baseline | 39.29 | 38.93 | 38.37 |
| Mean Change | 21.81 | 23.05 | 16.76 |
| Treatment Difference | 5.06 | 6.29 | |
| CI95% | (2.12, 7.99) | (3.03, 9.55) | |
| P-value | <.001d | <.001c | |
| MSQ Role Function Restrictive Domain Responders | |||
| N | 252 | 253 | 494 |
| Percentage, % | 64.3 | 64.8 | 54.1 |
| P-value | .003e | .002e | |
| MIDAS Total Score | |||
| N | 254 | 258 | 504 |
| Baseline | 62.46 | 69.17 | 68.66 |
| Mean Change | -20.27 | -17.02 | -11.53 |
| Treatment Difference | -8.74 | -5.49 | |
| CI95% | (-16.39, -1.08) | (-13.10, 2.12) | |
| P-value | .025e | >.05e | |
N = number of patients; CI95% = 95% confidence interval.
a Efficacy outcomes were evaluated across Months 1-3.
b Patient-reported outcomes were evaluated at Month 3. MSQ role function restrictive domain responders were defined as those with an improvement of ≥17.14 points for Chronic Migraine at Month 3.
c Statistically significant after adjustment for multiple comparisons.
d Not statistically significant after adjustment for multiple comparisons.
e Not adjusted for multiple comparisons.
In patients who failed one or more prophylactic treatments for efficacy reasons, the treatment difference for the reduction of mean monthly MHDs observed between galcanezumab 120 mg and placebo was -3.54 days (p<0.001) and between galcanezumab 240 mg and placebo -1.37 days (p<0.05). In patients failing two or more prophylactic treatments, the treatment difference was -4.48 days (p<0.001) between 120 mg and placebo and -1.86 days (p<0.01) between 240 mg and placebo.
Sixty-four percent of the patients had acute headache medication overuse at baseline. In these patients, the treatment difference observed between galcanezumab 120 mg and placebo and between galcanezumab 240 mg and placebo for the reduction of MHDs in these patients was respectively -2.53 days (p<0.001) and -2.26 days (p<0.001).
Long term efficacy
Efficacy was sustained for up to 1 year in an open-label study in which patients with either episodic or chronic migraine (with an average baseline of 10.6 monthly MHDs) received galcanezumab 120 mg/month (with an initial loading dose of 240 mg for the first month) or galcanezumab 240 mg/month. 77.8% of patients completed the treatment period. The overall mean reduction from baseline in the number of monthly MHDs averaged over the treatment phase was 5.6 days for the 120 mg dose group and 6.5 days for the 240 mg dose group. Over 72% of patients completing the study reported a 50% reduction in MHDs at month 12. In pooled data from studies EVOLVE-1 and EVOLVE-2, more than 19 % of the patients treated with galcanezumab maintained a ≥50% response from Month 1 to Month 6 versus 8% of the patients on placebo (p<0.001).
Phase 3 study in a population with previous failure to 2 to 4 migraine preventive medication categories
Study CONQUER, in episodic and chronic migraine patients that experienced previous failures to 2 to 4 prophylactic medication categories in the past 10 years, supports the main findings of the previous migraine efficacy studies, i.e. galcanezumab treatment led to a mean reduction in monthly migraine headache days (4.1 days compared to 1.0 days in the placebo group; p<.0001). Mean reduction in monthly migraine headache days was also observed within the subpopulations of episodic migraine (2.9 days for galcanezumab compared with 0.3 days for placebo; p<.0001) and chronic migraine (5.9 days for galcanezumab compared with 2.2 days for placebo; p<.0001).
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with galcanezumab in one or more subsets of the paediatric population in the prophylaxis of migraine headaches (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Based on a population pharmacokinetic (PK) analysis, following a loading dose of 240 mg the maximum serum concentration (Cmax) of galcanezumab was approximately 30 μg/mL (27% coefficient of variation, (CV)) and the time to Cmax was 5 days postdose.
Monthly doses of 120 mg or 240 mg achieved a steady-state Cmax (Cmax,ss) of approximately 28 μg/mL (35% CV) or 54 μg/mL (31% CV), respectively. The galcanezumab Cmax,ss at monthly doses of 120 mg is achieved after the 240 mg loading dose.
Injection site location (abdomen, thigh, buttocks and arm) did not significantly influence the absorption of galcanezumab.
Distribution
Based on a population PK analysis, the apparent volume of distribution of galcanezumab was 7.3 L.
Biotransformation
As a humanised IgG4 monoclonal antibody, galcanezumab is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
Elimination
Based on a population PK analysis, the apparent clearance of galcanezumab was approximately 0.008 L/hour and the half life of galcanezumab was 27 days.
Linearity/non-linearity
Galcanezumab exposure increases proportionally with dose. Based on a population PK analysis that included doses ranging from 5–300 mg, the rate of absorption, apparent clearance and apparent volume of distribution was independent of dose.
Age, sex, weight, race, ethnicity
No dose adjustment is needed on the basis of age (18 to 65 years), sex, weight, race or ethnicity as there was no clinically meaningful effect of these factors on the apparent clearance or apparent volume of distribution of galcanezumab.
Renal or hepatic impairment
Specific clinical pharmacology studies to evaluate the effects of renal impairment and hepatic impairment on the PK of galcanezumab have not been conducted. Renal elimination of IgG monoclonal antibody is low. Similarly, IgG monoclonal antibodies are mainly eliminated via intracellular catabolism and hepatic impairment is not expected to influence the clearance of galcanezumab. Based on a population PK analysis, bilirubin concentration or Cockcroft-Gault creatinine clearance (range: 24 to 308 mL/min) did not significantly influence the apparent clearance of galcanezumab.
Preclinical safety data
Non-clinical data revealed no special hazards for humans based on repeat-dose toxicity studies conducted in rats and cynomolgus monkeys and safety pharmacology evaluations conducted in cynomolgus monkeys at exposures approximately 10 to 80 times higher than clinical exposures in patients receiving 240 mg.
Nonclinical studies have not been conducted to evaluate the carcinogenic or mutagenic potential of galcanezumab. There is no evidence to suggest that chronic treatment with galcanezumab would increase the risk of carcinogenesis based on data from pharmacology and chronic toxicology studies with galcanezumab, as well as an assessment of the literature regarding CGRP.
No effects on fertility parameters such as oestrous cycle, sperm analysis, or mating and reproductive performance were observed in rats that were administered galcanezumab (exposures approximately 4 to 20 times the human exposure at 240 mg). In male fertility study, right testis weight was significantly reduced at exposures to 4 times the human exposure at 240 mg.
At Gestational Day 20, an increase in the number of foetuses and litters with short ribs and a decrease in the mean number of ossified caudal vertebrae occurred in the rat embryo-foetal toxicity development study at an exposure approximately 20 times the human exposure at 240 mg. These findings were noted at no maternal toxicity and were considered to be related to galcanezumab but non-adverse.
At Gestational Day 29, in rabbit embryo-foetal development toxicity study skull anomaly was found in one male foetus from mother treated with galcanezumab at an exposure approximately 33 times the human exposure at 240 mg.
In a juvenile toxicology study in which rats were administered galcanezumab twice weekly from Postnatal Day 21 through 90, systemic effects were limited to reversible, minimal, nonadverse decreases in total bone mineral content and bone mineral density at exposures approximately 50 times the human exposure at 240 mg.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.