EMTROC Film-coated tablet Ref.[50541] Active ingredients: Emtricitabine Tenofovir disoproxil
Source: Health Products Regulatory Authority (ZA) Revision Year: 2015 Publisher: Macleods Pharmaceuticals SA (Pty) Ltd, Stand 6 D & E, Growthpoint Business Park, Halfway House, Midrand, South Africa
4.4. Special warnings and precautions for use
LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS, INCLUDING FATAL CASES, HAVE BEEN REPORTED WITH THE USE OF NUCLEOSIDE ANALOGUES, ALONE OR IN COMBINATION WITH OTHER ANTIRETROVIRALS (SEE WARNINGS AND SPECIAL PRECAUTIONS).
EMTROC TABLETS IS NOT INDICATED FOR THE TREATMENT OF CHRONIC HEPATITIS B VIRUS (HBV) INFECTION AND THE SAFETYAND EFFICACY OF EMTROC TABLETS HAVE NOT BEEN ESTABLISHED IN PATIENTS COINFECTED WITH HBV AND HIV-1. SEVERE ACUTE EXACERBATIONS OF HEPATITIS B HAVE BEEN REPORTED IN PATIENTS WHO HAVE DISCONTINUED EMTRICITABINE (200 mg) OR TENOFOVIR. HEPATIC FUNCTION SHOULD BE MONITORED CLOSELY WITH BOTH CLINICALAND LABORATORY FOLLOWUP FOR AT LEAST SEVERAL MONTHS IN PATIENTS WHO DISCONTINUE EMTROC TABLETS AND ARE COINFECTED WITH HIV AND HBV. IF APPROPRIATE, INITIATION OF ANTI-HEPATITIS B THERAPY MAY BE WARRANTED (SEE WARNINGS AND SPECIAL PRECAUTIONS).
- There are no study results demonstrating the effect of EMTROC TABLETS on clinical progression of HIV-1.
- It is not recommended that EMTROC TABLETSbe used as a triple nucleoside regimen.
Lactic Acidosis / Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues, such as EMTROC TABLETS alone or in combination with other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Particular caution should be exercised when administering nucleoside analogues to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with EMTROC TABLETS should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations)
Clinical features are non-specific and include nausea, vomiting, abdominal pain, dyspnoea, fatigue and weight loss. Suspicious biochemical features include mild raised transaminase, raised lactate dehydrogenases (LDH) and/or creatine kinase. In patients with suspicious symptoms or −biochemistry, measure the venous lactate level (normal < 2 mmol/l) and respond as follows:
- Lactate 2-5 mmol/l: monitor regularly, and be alert for clinical signs
- Lactate 5-10 mmol/l without symptoms: monitor closely.
- Lactate 5-10 mmol/l with symptoms: STOP all therapy. Exclude other causes, (e.g. sepsis, uraemia, diabetic ketoacidosis, thyroxicosis, lymphoma).
- Lactate >10 mmol/l: STOP all therapy (80% mortality in case studies).
The above lactate levels may not be applicable to paediatric patients.
Patients with HIV and Hepatitis B Virus Coinfection
It is recommended that all patients with HIV be tested for the presence of hepatitis B virus (HBV) before initiating antiretroviral therapy. EMTROC TABLETS is not approved for the treatment of chronic HBV infection and the safety and efficacy of EMTROC TABLETS have not been established in patients coinfected with HBV and HIV. Severe acute exacerbations of hepatitis B have been reported in patients after the discontinuation of EMTROC TABLETS. Hepatic function should be closely monitored with both clinical and laboratory follow-up for at least several months in patients who discontinue EMTROC TABLETS and are coinfected with HIV and HBV. If appropriate, initiation of anti-hepatitis B therapy may be warranted.
Renal Impairment
Emtricitabine and tenofovir are principally eliminated by the kidney. Dosing interval adjustment of EMTROC TABLETS is recommended in all patients with creatinine clearance 30-49 mL/min, (see DOSAGE AND DIRECTIONS FOR USE). EMTROC TABLETS should not be administered to patients with creatinine clearance below 30 mL/min or patients requiring haemodialysis. Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphataemia), has been reported with the use of tenofovir (see SIDEEFFECTS). The majority of these cases occurred in patients with underlying systemic or renal disease, or in patients taking nephrotoxic agents, however, some cases occurred in patients without identified risk factor.
EMTROC TABLETS should be avoided with concurrent or recent use of a nephrotoxic agent. Patients at risk for, or with a history of, renal dysfunction and patients receiving concomitant nephrotoxic agents should be carefully monitored for changes in serum creatinine and phosphorus.
Medicine Interactions
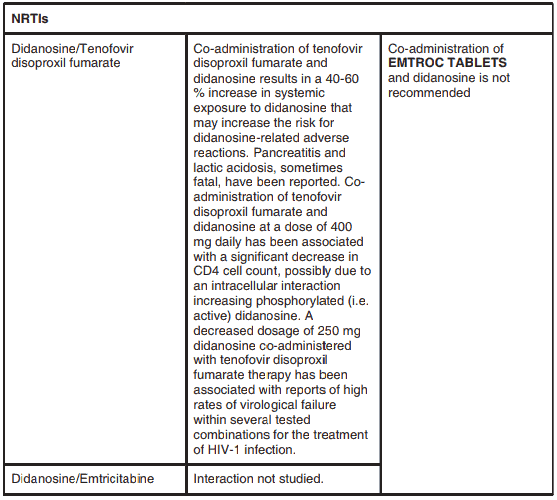
Coadministration of tenofovir disoproxil fumarate and didanosine is not recommended. Coadministration of tenofovir disoproxil fumarate and didanosine results in a 40-60% increase in systemic exposure to didanosine that may increase the risk for didanosine-related adverse reactions (see INTERACTIONS).
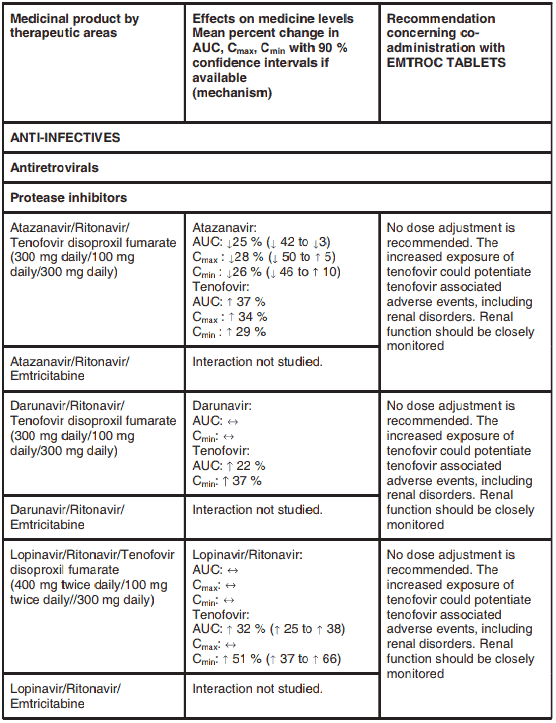
Atazanavir and lopinavir/ritonavir has been shown to increase tenofovir concentrations. The mechanism of this interaction is unknown. Patients receiving atazanavir and lopinavir/ritonavir and EMTROC TABLETS should be monitored for EMTROC TABLETS-associated adverse events. EMTROC TABLETS should be discontinued in patients who develop EMTROC TABLETSassociated adverse events.
Tenofovir decreases the AUC and Cmin of atazanavir. When coadministered with EMTROC TABLETS, it is recommended that atazanavir 300 mg is given with ritonavir 100 mg. Atazanavir without ritonavir should not be coadministered with EMTROC TABLETS.
Emtricitabine and tenofovir disoproxil fumarate: Since emtricitabine and tenofovir are primarily eliminated by the kidney, coadministration of EMTROC TABLETS with medicines that reduce renal function or compete for active tubular secretion may increase serum concentration of emtricitabine, tenofovir and/or other renally eliminated medicines. Some examples include, but are not limited to acyclovir, adefovir dipivoxil, cidofovir, ganciclovir, and valganciclovir. EMTROC TABLETS is a fixed-dose combination of emtricitabine and tenofovir disoproxil fumarate.
EMTROC TABLETS should not be coadministered with emtricitabine, or tenofovir. Due to similarities between emtricitabine and lamivudine, EMTROC TABLETS should not be coadministered with other medicines containing lamivudine and zidovudine coformulation, lamivudine for HIV, lamivudine for HBV, abacavir sulfate and lamivudine coformulation or abacavir sulfate, lamivudine and zidovudine coformulation.
Opportunistic infections
Patients receiving EMTROC TABLETS may continue to develop opportunistic infections and other complications of HIV infection, and therefore should remain under close observation by medical practitioners experienced in the treatment of patients with associated HIV disease.
The risk of HIV transmission to others
Patients should be advised that current antiretroviral therapy, including EMTROC TABLETS, has not been proven to prevent the risk of transmission of HIV to others through sexual contact or blood contamination. Appropriate precautions should continue to be employed.
Bone effects
EMTROC TABLETS decreases bone mineral density. Bone monitoring should be considered for HIV infected patients who have a history of pathologic bone fracture or are at risk for osteopenia. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial for all patients. If bone abnormalities are suspected then appropriate consultation should be obtained.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequencesofthese eventsare currently unknown.A causalrelationshiphas not beenestablished.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including EMTROC TABLETS. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment.
Paediatric Use
Safety and effectiveness in paediatric patients have not been established.
Geriatric Use
Dose selection for the elderly patients should be cautious, keeping in mind the greater frequency of decreased hepatic,renal,or cardiac function, and ofconcomitant disease orother medicinetherapy.
Lactose intolerance
EMTROC TABLETS contain lactose and should not be given to patients with known hereditary problems, or a history of galactose intolerance, Lapp-lactase deficiency or glucose-galactose malabsorption.
4.5. Interaction with other medicinal products and other forms of interaction
As EMTROC TABLETS contains emtricitabine and tenofovir disoproxil fumarate, any interactions that have been identified with these agents individually may occur with EMTROC TABLETS. The steady-state pharmacokinetics of emtricitabine and tenofovir were unaffected when emtricitabine and tenofovir disoproxil fumarate were administered together versus each medicinal product dosed alone.
In vitro and clinical pharmacokinetic interaction studies have shown the potential for CYP450 mediated interactions involving emtricitabine and tenofovir disoproxil fumarate with other medicinal products is low.
Concomitant use not recommended:
Due to similarities with emtricitabine, EMTROC TABLETS should not be administered concomitantly with other cytidine analogues, such as lamivudine. As a fixed combination, EMTROC TABLETS should not be administered concomitantly with other medicinal products containing any of the components, emtricitabine or tenofovir disoproxil fumarate. EMTROC TABLETSshould not be administered concomitantly with adefovir dipivoxil.
Didanosine: The co-administration of EMTROC TABLETSand didanosine is not recommended. Renally eliminated medicinal products: Since emtricitabine and tenofovir are primarily eliminated by the kidneys, co-administration of EMTROC TABLETS with medicinal products that reduce renal function or compete for active tubular secretion (e.g. cidofovir) may increase serum concentrations of emtricitabine, tenofovir and/or the co-administered medicinal products. Use of EMTROC TABLETS should be avoided with concurrent or recent use of a nephrotoxic medicinal product. Some examples include, but are not limited to, aminoglycosides, amphotericin B, foscarnet, ganciclovir, pentamidine, vancomycin, cidofovir or interleukin-2.
Other interactions
Interactions between the components of EMTROC TABLETS and protease inhibitors and nucleoside reverse transcriptase inhibitors, are listed in Table 2 below (increase is indicated as “↑”, decrease as “↓”, no change as “↔”). If available, 90% confidence intervals are shown in parentheses.
Table 2. Interactions between the individual components of EMTROC TABLETS and other medicinal products:
Studies conducted with other medicinal products
Emtricitabine: In vitro, emtricitabine did not inhibit metabolism mediated by any of the following human CYP450 isoforms: 1A2, 2A6, 2B6, 2C9, 2C19, 2D6 and 3A4. Emtricitabine did not inhibit the enzyme responsible for glucuronidation.
There are no clinically significant pharmacokinetic interactions when emtricitabine is coadministered with indinavir, zidovudine, stavudine or famciclovir.
Tenofovir disoproxil fumarate: Co-administration of lamivudine, indinavir, efavirenz, nelfinavir or saquinavir (ritonavir boosted), methadone, ribavirin, rifampicin, adefovir dipivoxil or the hormonal contraceptive norgestimate/ethinyl oestradiol with tenofovir disoproxil fumarate did not result in any clinically significant pharmacokinetic interaction.
Atazanavir and lopinavir/ritonavir has been shown to increase tenofovir concentrations. The mechanism of this interaction is unknown.
4.6. Pregnancy and lactation
Safety and use in pregnancy and lactation has not been established.
It is not known whether EMTROC TABLETSis excreted in human milk.
Breast-feeding Mothers: HIV-1 infected mothers should not breast-feed their infants to avoid risking postnatal transmission of HIV-1. Because of both the potential for HIV-1 transmission and the potential for serious adverse reactions in breast-fed infants, mothers should be instructed not to breast-feed if they are receiving EMTROC TABLETS.
4.7. Effects on ability to drive and use machines
EMTROC TABLETS may cause dizziness, impaired concentration, and/or drowsiness. Patients should be instructed that if they experience these symptoms they should avoid potentially hazardous tasks such as driving or operating machinery.
4.8. Undesirable effects
EMTRICITABINE (200 mg)
Nervous system disorders
Frequent: Headache
Frequency not known: Asthenia, dizziness, neuropathy, peripheral neuritis, paraesthesia.
Psychiatric disorders
Frequency not known: Sleep disturbances (abnormal dreams, insomnia), depressive disorder
Gastrointestinal disorders
Frequent: Nausea, vomiting, diarrhoea
Frequency not known: Abdominal pain, dyspepsia.
Skin and subcutaneous tissue disorders
Frequent: Rash event (including rash, pruritus, maculopapular rash, urticaria, vesiculobullous rash, pustular rash and allergic reaction).
Frequency not known: Skin discolouration, manifested by hyperpigmentation on the palm and/or soles.
Musculoskeletal and connective tissue disorders
Frequency not known: Arthralgia, myalgia.
Blood and lymphatic system disorders
Frequency not known: Neutropenia, anaemia.
Renal and urinary disorders
Frequent: Elevation of creatinine kinase.
Respiratory, thoracic and mediastinal disorders
Frequency not known: Increased cough and rhinitis.
Hepatobiliary disorders
Frequency not known: Raised liver enzyme concentrations and hyperbilirubinaemia.
Metabolism and nutrition disorders
Frequency not known: Lactic acidosis, usually associated with severe hepatomegaly and steatosis, hypertriglyceridaemia, hyperglycaemia.
TENOFOVIR (300 mg)
Gastrointestinal disorders
Frequent: Nausea, vomiting, diarrhoea, abdominal pain, flatulence, dyspepsia and anorexia.
Less frequent: Raised serum amylase concentration, pancreatitis.
Frequency not known: Abdominal pain, dyspepsia.
Nervous system disorders
Frequency not known: Headache, asthenia, dizziness, peripheral neuropathy (including peripheral neuritis, neuropathy).
Psychiatric disorders
Frequency not known: Depression, anxiety.
Skin and subcutaneous tissue disorders
Frequency not known: Skin rashes (including rash, pruritus, maculopapular rash, urticaria, vesiculobullous rash, pustular rash).
Musculoskeletal and connective tissue disorders
Frequency not known: Back pain, arthralgia, myalgia.
Respiratory, thoracic and mediastinal disorders
Frequency not known: Chest pain, pneumonia, dyspnoea.
Blood and lymphatic system disorders
Frequency not known: Neutropenia, haematuria.
Hepatobiliary disorders
Frequency not known: Raised liver enzymes and hepatitis.
Metabolism and nutrition disorders
Frequent: Hypophosphataemia
Frequency not known: Lactic acidosis, usually associated with sever hepatomegaly and steatosis, hypertriglyceridaemia, hyperglycemia
Renal and urinary disorders
Frequency not known: Increased creatinine levels, nephritis, nephrogenic diabetes insipidus, renal impairment, acute renal failure, and effects on the renal proximal tubules including Fanconi syndrome, renal failure, proximal tubulopathy, proteinuria, acute tubular necrosis
Immune system disorders
Frequency not known: Allergy, allergic reaction
General disorders
Frequency not known: Fever, sweating, weight loss.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.