ENDOMETRIN Vaginal insert Ref.[10804] Active ingredients: Progesterone
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
ENDOMETRIN (progesterone) Vaginal Insert contains micronized progesterone. ENDOMETRIN is supplied with polyethylene vaginal applicators.
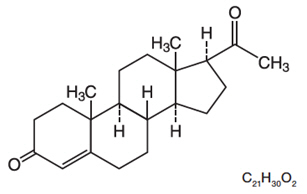
The active ingredient, progesterone, is present in 100 mg amount along with other excipients. The chemical name for progesterone is pregn-4-ene-3,20-dione. It has an empirical formula of C21H30O2 and a molecular weight of 314.5. Progesterone exists in two polymorphic forms. The form used in ENDOMETRIN, the alpha-form, has a melting point of 127-131°C.
The structural formula is:
Each ENDOMETRIN Vaginal Insert delivers 100 mg of progesterone in a base containing lactose monohydrate, polyvinylpyrrolidone, adipic acid, sodium bicarbonate, sodium lauryl sulfate, magnesium stearate, pregelatinized starch, and colloidal silicon dioxide.
| Dosage Forms and Strengths |
|---|
|
100 mg vaginal insert is a white to off-white oblong-shaped tablet debossed with "FPI" on one side and "100" on the other side. |
| How Supplied |
|---|
|
Each ENDOMETRIN Vaginal Insert is a white to off-white oblong-shaped insert debossed with "FPI" on one side and "100" on the other side. Each ENDOMETRIN (progesterone) Vaginal Insert, 100 mg, is packed individually in a sealed foil pouch. These pouches are available in cartons packed:
MANUFACTURED FOR: FERRING PHARMACEUTICALS INC., PARSIPPANY, NJ 07054 |
Drugs
| Drug | Countries | |
|---|---|---|
| ENDOMETRIN | Australia, Canada, Ecuador, Hong Kong, Israel, Mexico, Nigeria, Singapore, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.