ENSTILAR Foam Ref.[50952] Active ingredients: Betamethasone Calcipotriol
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
Enstilar Foam contains calcipotriene hydrate and betamethasone dipropionate. It is for topical use only.
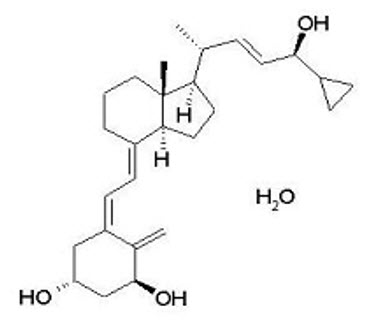
Calcipotriene Hydrate
Calcipotriene hydrate is a synthetic vitamin D analog and has the chemical name 9,10-secochola-5,7,10(19),22-tetraene-1,3,24-triol,24-cyclo-propyl-,monohydrate, (1α,3β,5Z,7E,22E,24S) with the empirical formula C27H40O3∙H2O), a molecular weight of 430.6, and the following structural formula (calcipotriene hydrate is a white to almost white, crystalline compound):
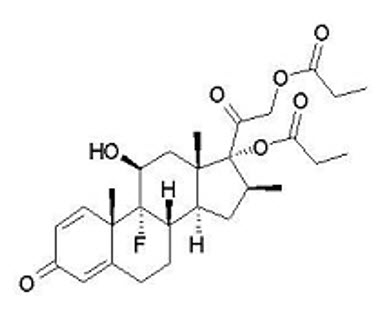
Betamethasone Dipropionate
Betamethasone dipropionate is a synthetic corticosteroid and has the chemical name pregna-1,4-diene-3,20-dione-9-fluoro-11-hydroxy-16-methyl-17,21-bis(1-oxypropoxy)-(11β,16β), with the empirical formula C28H37FO7, a molecular weight of 504.6, and the following structural formula (betamethasone dipropionate is a white to almost white, crystalline powder):
Enstilar Foam
Each gram of Enstilar Foam contains 50 mcg of calcipotriene (equivalent to 52.2 mcg of calcipotriene hydrate) and 0.643 mg of betamethasone dipropionate (equivalent to 0.5 mg of betamethasone) in a base of white petrolatum, polyoxypropylene stearyl ether, mineral oil, all-rac-alpha-tocopherol, and butylhydroxytoluene. Enstilar Foam is a white to off-white opalescent liquid in a pressurized aluminum spray can with a continuous valve and actuator. The propellants used in Enstilar Foam are dimethyl ether and butane. At administration, the product is a white to off-white foam after evaporation of the propellants. Enstilar Foam has the appearance of a non-expanding foam that gradually collapses after spraying.
| Dosage Forms and Strengths |
|---|
|
Enstilar Foam: 0.005%/0.064% - each gram contains 50 mcg calcipotriene and 0.643 mg of betamethasone dipropionate in a white to off-white opalescent liquid in a pressurized aluminum spray can with a continuous valve and actuator. At administration the product is a white to off-white foam after evaporation of the propellants. |
| How Supplied |
|---|
|
Enstilar (calcipotriene and betamethasone dipropionate) Foam, 0.005%/0.064% is a white to off-white opalescent liquid in a pressurized aluminum spray can with a continuous valve and actuator. At administration, the product is a white to off-white foam after evaporation of the propellants. It is available as:
Manufactured by: LEO Laboratories Ltd., 285 Cashel Road, Dublin 12, Ireland Distributed by: LEO Pharma Inc., Madison, NJ 07940, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| ENSTILAR | Austria, Australia, Canada, Cyprus, Estonia, Spain, Finland, France, Hong Kong, Croatia, Ireland, Israel, Lithuania, Malta, Netherlands, New Zealand, Poland, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.