ENTERO VU 24% Oral suspension Ref.[50368] Active ingredients: Barium sulfate
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
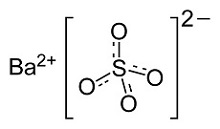
ENTERO VU 24% (barium sulfate) is a radiographic contrast agent that is supplied as a suspension (24% w/v) for oral administration. The active ingredient barium sulfate is designated chemically as BaSO4 with a molecular weight of 233.4 g/mol, a density of 4.5 g/cm³, and the following chemical structure
ENTERO VU 24% contains the following excipients: acacia, carrageenan, citric acid, methylcellulose, natural and artificial blueberry flavor, polysorbate 80, potassium chloride, potassium sorbate, purified water, saccharin sodium, simethicone emulsion, sodium benzoate, sodium citrate, sorbitol solution, and xanthan gum.
| Dosage Forms and Strengths |
|---|
|
Oral suspension: barium sulfate (24% w/v) supplied in a single-dose white HDPE plastic bottle as a ready-to-use suspension for oral administration. Each bottle contains 600 mL of suspension. |
| How Supplied |
|---|
|
ENTERO VU 24% (barium sulfate) is supplied as a suspension (24% w/v) in a single-dose HDPE plastic bottle containing 600 mL of barium sulfate suspension (24% w/v). Provided as: 6 × 600 mL bottles (NDC 32909-146-06) Manufactured by EZEM Canada Inc, Anjou (Quebec) Canada H1J 2Z4 For Bracco Diagnostics Inc., Monroe Township, NJ 08831 |
Drugs
| Drug | Countries | |
|---|---|---|
| ENTERO VU | United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.