EPCLUSA Film-coated tablet Ref.[10464] Active ingredients: Sofosbuvir Sofosbuvir and Velpatasvir Velpatasvir
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Gilead Sciences Ireland UC, Carrigtohill, County Cork, T45 DP77, Ireland
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
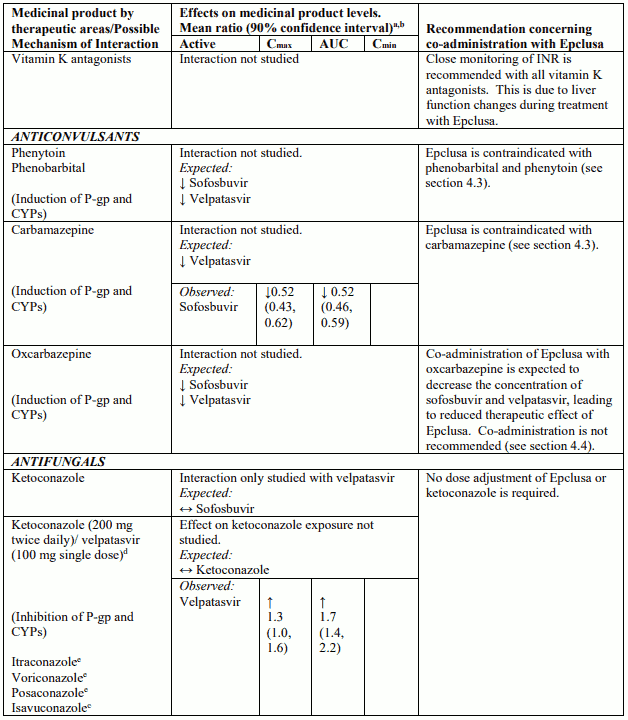
Medicinal products that are strong P-glycoprotein (P-gp) and/or strong cytochrome P450 (CYP) inducers (carbamazepine, phenobarbital, phenytoin, rifampicin, rifabutin and St. John’s wort) (see section 4.5).
4.4. Special warnings and precautions for use
Epclusa should not be administered concurrently with other medicinal products containing sofosbuvir.
Severe bradycardia and heart block
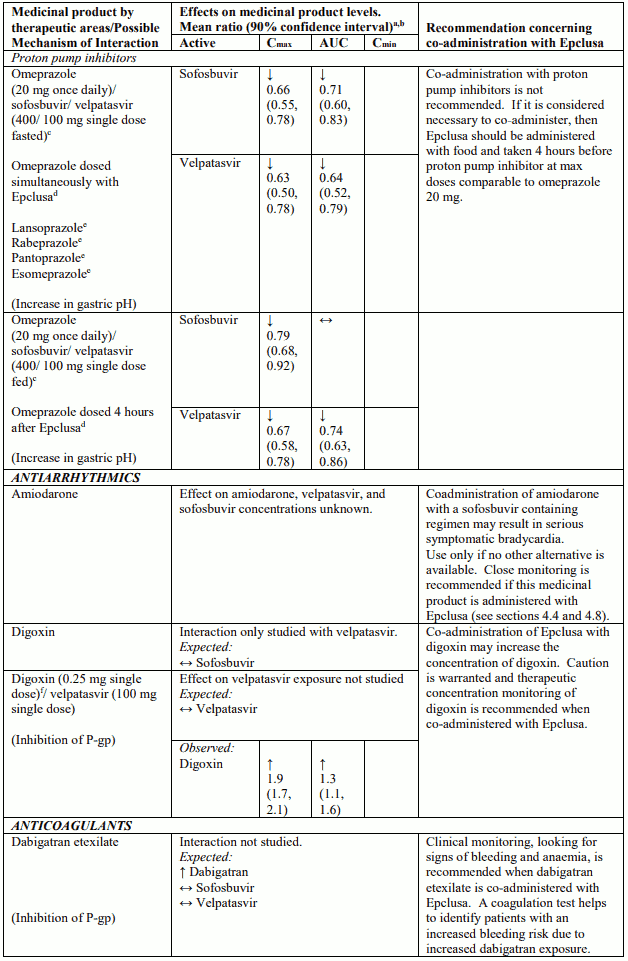
Life-threatening cases of severe bradycardia and heart block have been observed when sofosbuvircontaining regimens are used in combination with amiodarone. Bradycardia has generally occurred within hours to days, but cases with a longer time to onset have been observed mostly up to 2 weeks after initiating HCV treatment.
Amiodarone should only be used in patients on Epclusa when other alternative anti-arrhythmic treatments are not tolerated or are contraindicated.
Should concomitant use of amiodarone be considered necessary, it is recommended that patients undergo cardiac monitoring in an in-patient setting for the first 48 hours of co-administration, after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.
Due to the long half-life of amiodarone, cardiac monitoring as outlined above should also be carried out for patients who have discontinued amiodarone within the past few months and are to be initiated on Epclusa.
All patients with concurrent or recent use of amiodarone should be warned of the symptoms of bradycardia and heart block and should be advised to seek medical advice urgently should they experience them.
HCV / HBV (hepatitis B virus) co-infection
Cases of hepatitis B virus (HBV) reactivation, some of them fatal, have been reported during or after treatment with direct-acting antiviral medicinal products. HBV screening should be performed in all patients before initiation of treatment. HBV/HCV co-infected patients are at risk of HBV reactivation, and should, therefore, be monitored and managed according to current clinical guidelines.
Patients who have previously failed therapy with an NS5A-containing regimen
There are no clinical data to support the efficacy of sofosbuvir/velpatasvir for the treatment of patients who have failed treatment with a regimen containing another NS5A inhibitor. However, on the basis of NS5A resistance associated variants (RAVs) typically seen in patients who have failed therapy with other NS5A inhibitor containing regimens, the in vitro pharmacology of velpatasvir, and the outcomes of sofosbuvir/velpatasvir treatment in NS5A-naïve patients with baseline NS5A RAVs enrolled into the ASTRAL-studies, treatment with Epclusa + RBV for 24 weeks can be considered for patients who have failed therapy on an NS5A-containing regimen and who are deemed at high risk for clinical disease progression and who do not have alternative treatment options.
Renal impairment
Safety data are limited in patients with severe renal impairment (eGFR <30 mL/min/1.73 m²) and ESRD requiring haemodialysis. Epclusa can be used in these patients with no dose adjustment when no other relevant treatment options are available (see sections5.1 and 5.2). When Epclusa is used in combination with ribavirin refer also to the Summary of Product Characteristics for ribavirin for patients with creatinine clearance <50 mL/min (see section 5.2).
Use with moderate P-gp inducers and/or moderate CYP inducers
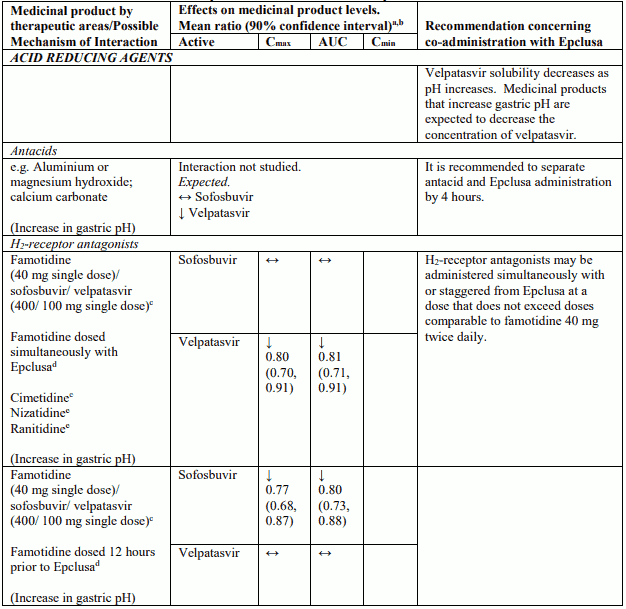
Medicinal products that are moderate P-gp and/or moderate CYP inducers (e.g. efavirenz, modafinil, oxcarbazepine or rifapentine) may decrease sofosbuvir or velpatasvir plasma concentrations leading to reduced therapeutic effect of Epclusa. Co-administration of such medicinal products with Epclusa is not recommended (see section 4.5).
Use with certain HIV antiretroviral regimens
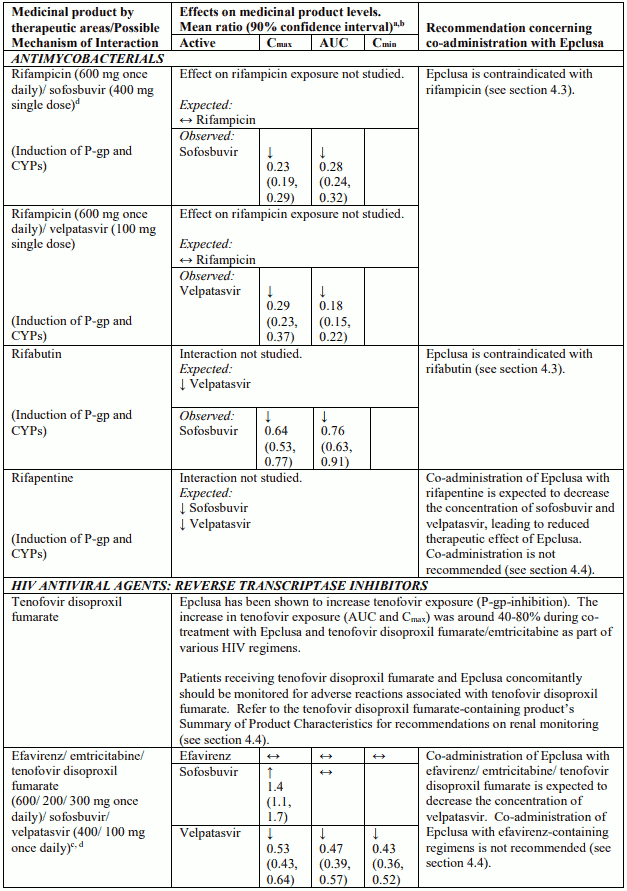
Epclusa has been shown to increase tenofovir exposure, especially when used together with an HIV regimen containing tenofovir disoproxil fumarate and a pharmacokinetic enhancer (ritonavir or cobicistat). The safety of tenofovir disoproxil fumarate in the setting of Epclusa and a pharmacokinetic enhancer has not been established. The potential risks and benefits associated with co-administration of Epclusa with the fixed-dose combination tablet containing elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate or tenofovir disoproxil fumarate given in conjunction with a boosted HIV protease inhibitor (e.g. atazanavir or darunavir) should be considered, particularly in patients at increased risk of renal dysfunction. Patients receiving Epclusa concomitantly with elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor should be monitored for tenofovir-associated adverse reactions. Refer to tenofovir disoproxil fumarate, emtricitabine/tenofovir disoproxil fumarate, or elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate Summary of Product Characteristics for recommendations on renal monitoring.
Use in diabetic patients
Diabetics may experience improved glucose control, potentially resulting in symptomatic hypoglycaemia, after initiating HCV direct-acting antiviral treatment. Glucose levels of diabetic patients initiating direct-acting antiviral therapy should be closely monitored, particularly within the first 3 months, and their diabetic medication modified when necessary. The physician in charge of the diabetic care of the patient should be informed when direct-acting antiviral therapy is initiated.
CPT Class C cirrhosis
Safety and efficacy of Epclusa has not been assessed in patients with CPT Class C cirrhosis (see sections 4.8 and 5.1).
Liver transplant patients
The safety and efficacy of Epclusa in the treatment of HCV infection in patients who are post-liver transplant have not been assessed. Treatment with Epclusa in accordance with the recommended posology (see section 4.2) should be guided by an assessment of the potential benefits and risks for the individual patient.
Excipients
This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
As Epclusa contains sofosbuvir and velpatasvir, any interactions that have been identified with these active substances individually may occur with Epclusa.
Potential for Epclusa to affect other medicinal products
Velpatasvir is an inhibitor of drug transporter P-gp, breast cancer resistance protein (BCRP), organic anion-transporting polypeptide (OATP) 1B1 and OATP1B3. Co-administration of Epclusa with medicinal products that are substrates of these transporters may increase the exposure of such medicinal products. See Table 4 for examples of interactions with sensitive substrates of P-gp (digoxin), BCRP (rosuvastatin), and OATP (pravastatin).
Potential for other medicinal products to affect Epclusa
Sofosbuvir and velpatasvir are substrates of drug transporters P-gp and BCRP. Velpatasvir is also a substrate of drug transporter OATP1B. In vitro, slow metabolic turnover of velpatasvir by CYP2B6, CYP2C8 and CYP3A4 was observed. Medicinal products that are strong inducers of P-gp and/or strong inducers of CYP2B6, CYP2C8, or CYP3A4 (e.g. carbamazepine, phenobarbital and phenytoin, rifampicin, rifabutin and St. John’s wort) may decrease plasma concentrations of sofosbuvir or velpatasvir leading to reduced therapeutic effect of sofosbuvir/velpatasvir. The use of such medicinal products with Epclusa is contraindicated (see section 4.3). Medicinal products that are moderate P-gp inducers and/or moderate CYP inducers (e.g. efavirenz, modafinil, oxcarbazepine or rifapentine) may decrease sofosbuvir or velpatasvir plasma concentration leading to reduced therapeutic effect of Epclusa. Co-administration with such medicinal products is not recommended with Epclusa (see section 4.4). Co-administration with medicinal products that inhibit P-gp or BCRP may increase sofosbuvir or velpatasvir plasma concentrations. Medicinal products that inhibit OATP, CYP2B6, CYP2C8, or CYP3A4 may increase plasma concentration of velpatasvir. Clinically significant medicinal product interactions with Epclusa mediated by P-gp, BCRP, OATP, or CYP450 inhibitors are not expected; Epclusa may be co-administered with P-gp, BCRP, OATP and CYP inhibitors.
Patients treated with vitamin K antagonists
As liver function may change during treatment with Epclusa, a close monitoring of International Normalised Ratio (INR) values is recommended.
Impact of DAA therapy on drugs metabolized by the liver
The pharmacokinetics of medicinal products that are metabolized by the liver (e.g. immunosuppressive medicinal products such as calcineurin inhibitors) may be impacted by changes in liver function during DAA therapy, related to clearance of HCV.
Interactions between Epclusa and other medicinal products
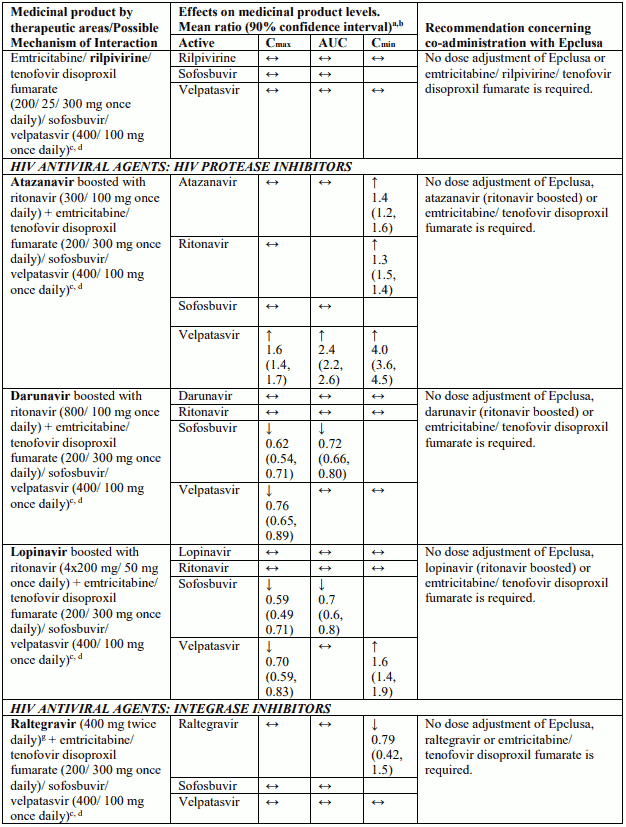
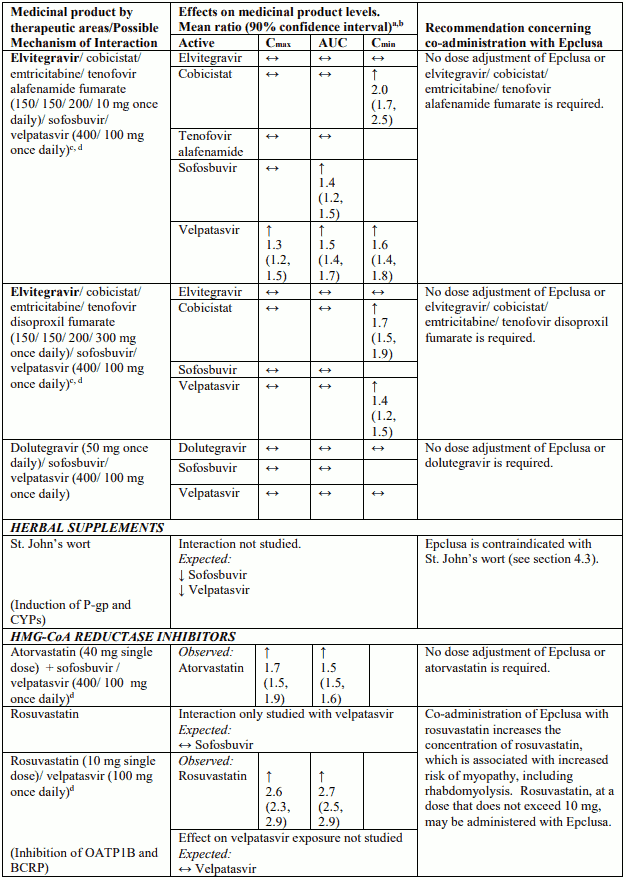
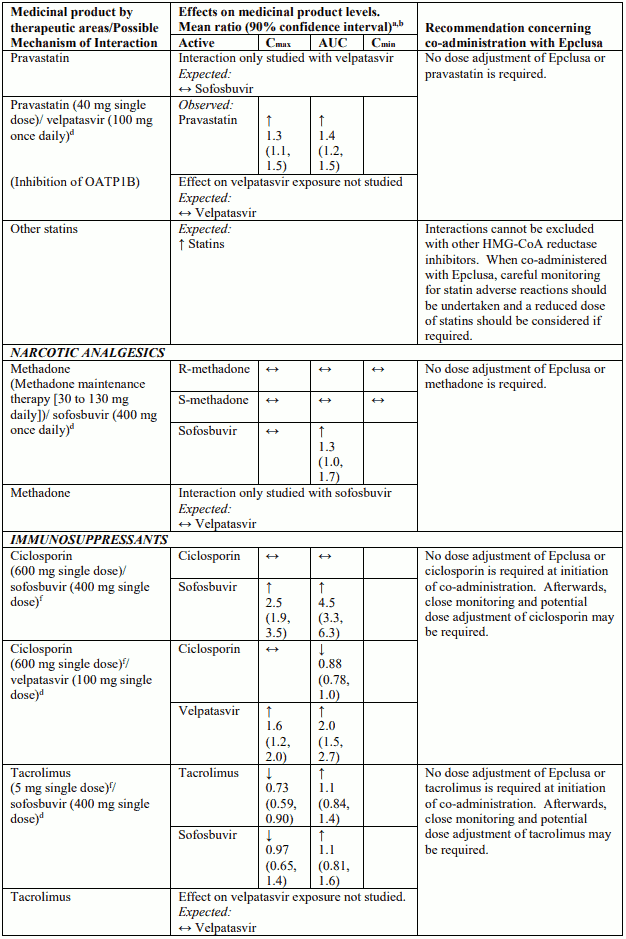
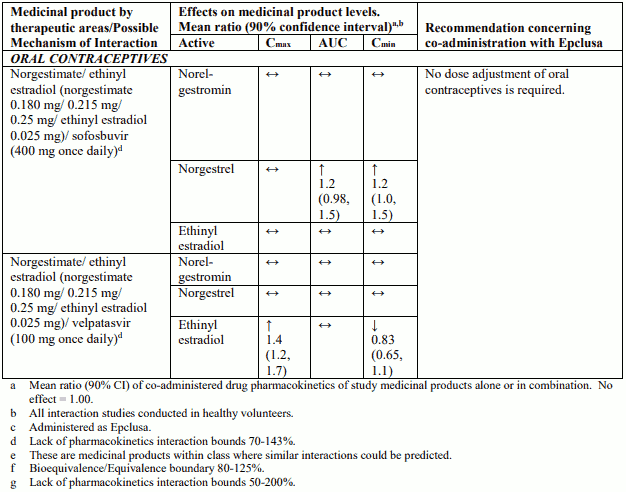
Table 4 provides a listing of established or potentially clinically significant medicinal product interactions (where 90% confidence interval [CI] of the geometric least-squares mean [GLSM] ratio were within “↔”, extended above “↑”, or extended below “↓” the predetermined interaction boundaries). The medicinal product interactions described are based on studies conducted with either sofosbuvir/velpatasvir or velpatasvir and sofosbuvir as individual agents, or are predicted medicinal product interactions that may occur with sofosbuvir/velpatasvir. The table is not all-inclusive.
Table 4. Interactions between Epclusa and other medicinal products:
4.6. Fertility, pregnancy and lactation
Pregnancy
There are no or limited amount of data (less than 300 pregnancy outcomes) from the use of sofosbuvir, velpatasvir or Epclusa in pregnant women.
Sofosbuvir
Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity (see section 5.3).
It has not been possible to fully estimate exposure margins achieved for sofosbuvir in the rat relative to the exposure in humans at the recommended clinical dose (see section 5.3).
Velpatasvir
Animal studies have shown a possible link to reproductive toxicity (see section 5.3).
As a precautionary measure, Epclusa use is not recommended during pregnancy.
Breast-feeding
It is unknown whether sofosbuvir, metabolites of sofosbuvir or velpatasvir are excreted in human milk.
Available pharmacokinetic data in animals have shown excretion of velpatasvir and metabolites of sofosbuvir in milk.
A risk to the newborns/infants cannot be excluded. Therefore, Epclusa should not be used during breast-feeding.
Fertility
No human data on the effect of Epclusa on fertility are available. Animal studies do not indicate harmful effects of sofosbuvir or velpatasvir on fertility.
If ribavirin is co-administered with Epclusa, refer to the Summary of Product Characterisitics for ribavirin for detailed recommendations regarding pregnancy, contraception, and breast-feeding.
4.7. Effects on ability to drive and use machines
Epclusa has no or negligible influence on the ability to drive and use machines.
4.8. Undesirable effects
Summary of the safety profile
The safety profile of Epclusa has been determined in pooled Phase 3 clinical studies of patients with genotype 1, 2, 3, 4, 5 or 6 HCV infection and in the postmarketing setting. No adverse drug reactions to Epclusa were identified from clinical studies. In the postmarketing setting, cases of severe bradycardia and heart block have been observed when SOF-containing products are used in combination with amiodarone, and HBV reactivation has been observed in patients coinfected with HCV/HBV following treatment with DAAs (see section 4.4).
Tabulated summary of adverse reactions
Assessment of adverse reactions for Epclusa is based on safety data from clinical studies and postmarketing experience. All adverse reactions are presented in Table 5. The adverse reactions are listed below by system organ class and frequency. Frequencies are defined as follows: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1000 to <1/100); rare (≥1/10,000 to <1/1000) or very rare (<1/10,000).
Table 5. Adverse drug reactions identified with Epclusa:
| Frequency | Adverse drug reaction |
|---|---|
| Gastrointestinal disorders | |
| Very common | vomitinga |
| Skin and subcutaneous tissue disorders | |
| Common | rashb |
| Uncommon | angioedemab |
a Adverse reaction was observed in paediatric patients aged 3 to <6 years
b Adverse reaction identified through post-marketing surveillance for sofosbuvir/velpatasvir-containing products
Description of selected adverse reactions
Cardiac arrhythmias
Cases of severe bradycardia and heart block have been observed when sofosbuvir-containing regimens are used in combination with amiodarone and/or other medicinal products that lower heart rate (see sections 4.4 and 4.5).
Skin disorders
Frequency not known: Stevens-Johnson syndrome
Paediatric population
The adverse reactions observed were consistent with those observed in clinical studies of Epclusa in adults. Vomiting was observed as a very common adverse drug reaction to Epclusa in paediatric patients aged 3 to <6 years. The safety assessment of Epclusa in paediatric patients aged 3 years and older is based on data from a Phase 2, open-label clinical study (study 1143) that enrolled 216 patients who were treated with sofosbuvir/velpatasvir for 12 weeks.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.