ESMOLOL HYDROCHLORIDE Powder for concentrate solution Ref.[7954] Active ingredients: Esmolol
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Orpha-Devel Handels und Vertriebs GmbH, Wintergasse 85/1B, A-3002 Purkersdorf, Austria
Therapeutic indications
ESMOLOL HYDROCHLORIDE 2500 mg powder is indicated for supraventricular tachycardia (except for pre- excitation syndromes), and for the rapid control of ventricular rate in patients with atrial fibrillation or atrial flutter in perioperative, postoperative, or other circumstances where short-term control of the ventricular rate with a short acting agent is desirable.
ESMOLOL HYDROCHLORIDE 2500 mg powder is also indicated for tachycardia and hypertension occurring in the perioperative phase and non-compensatory sinus tachycardia where, in physician’s judgement the rapid heart rate requires specific intervention.
ESMOLOL HYDROCHLORIDE 2500 mg powder is not indicated for use in children aged up to 18 years (see section 4.2).
ESMOLOL HYDROCHLORIDE 2500 mg powder is not intended for use in chronic settings.
Posology and method of administration
ESMOLOL HYDROCHLORIDE 2500 mg powder for concentrate for solution for infusion MUST NOT BE ADMINISTERED WITHOUT RECONSTITUTION/DILUTION.
The reconstituted/diluted solution for infusion must be used immediately after opening (see sections 4.4 and 6).
The administration of incorrect reconstituted/diluted ESMOLOL HYDROCHLORIDE 2500 mg powder may result in death (see section 4.4).
Posology
Supraventricular tachyarrhythmia
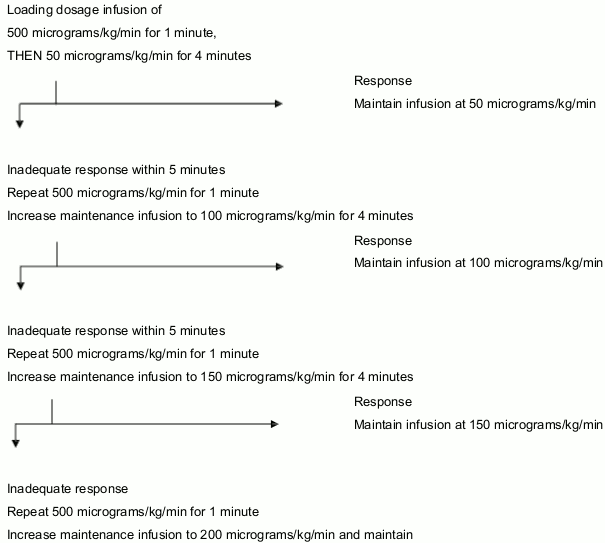
The dosage of ESMOLOL HYDROCHLORIDE 2500 mg powder should be titrated individually. A starting dose is required, followed by a maintenance dosage.
The effective dose of ESMOLOL HYDROCHLORIDE 2500 mg powder is within the range of 50 to 200 micrograms/kg/min, although doses as high as 300 micrograms/kg/min have been used. In a few patients the average effective dosage of 25 micrograms/kg/min has been adequate.
Flow chart for initiation and maintenance of treatment:
Inadequate response
Repeat 500 micrograms/kg/min for 1 minute
Increase maintenance infusion to 200 micrograms/kg/min and maintain
As the desired heart rate or safety end-point (e.g. lowered blood pressure) is approached, OMIT the loading infusion and reduce the incremental dose in the maintenance infusion from 50 micrograms/kg/min to 25 micrograms/kg/min or lower. If necessary, the interval between the titration steps may be increased from 5 to 10 minutes.
NB: Maintenance doses above 200 micrograms/kg/min have not been shown to have significantly increased benefits, and the safety of doses above 300 micrograms /kg/min has not been studied.
In the event of an adverse reaction, the dosage of ESMOLOL HYDROCHLORIDE 2500 mg powder may be reduced or discontinued. Pharmacological adverse reactions should resolve within 30 minutes.
If a local infusion site reaction develops, an alternative infusion site should be used and caution should be taken to prevent extravasation.
The administration of ESMOLOL HYDROCHLORIDE 2500 mg powder infusions for longer than 24 hours has not been thoroughly evaluated. Infusion durations greater than 24 hours should only be used with caution.
Abrupt discontinuation of ESMOLOL HYDROCHLORIDE 2500 mg powder in patients has not been reported to produce the withdrawal effects which may occur with abrupt withdrawal of beta-blockers following chronic use in coronary artery disease (CAD) patients. However, caution should still be used in discontinuing ESMOLOL HYDROCHLORIDE 2500 mg powder infusions abruptly in CAD patients.
Periopertatuve tachycardia and hypertension
For perioperative tachycardia and hypertension the dosing regimen may vary as follows:
a) For the intraoperative treatment – during anaesthesia when immediate control is required, a bolus injection of 80 mg is given over 15 to 30 seconds, followed by a 150 micrograms/kg/min infusion. Titrate the infusion rate as required up to 300 micrograms/kg/min.
b) Upon awakening from anaesthesia administer an infusion of 500 micrograms/kg/min for up to 4 minutes followed by an infusion of 300 micrograms/kg/min.
c) For postoperative situations when time for titration is available give the 500 micrograms/kg/min loading dose over one minute before each titration step to produce a rapid onset of action. Use titration steps of 50, 100, 150, 200, 250 and 300 micrograms/kg/min given over four minutes, stopping at the desired therapeutic effect.
Replacement of ESMOLOL HYDROCHLORIDE 2500 mg powder therapy by alternative drugs
After patients achieve an adequate control of the heart rate and a stable clinical status, transition to alternative drugs (such as antiarrhytmics or calcium antagonists) may be accomplished.
Reducing the dosage:
When ESMOLOL HYDROCHLORIDE 2500 mg powder is to be replaced by alternative drugs, the physician should carefully consider the labelling of the alternative drug selected and reduce the dosage of ESMOLOL HYDROCHLORIDE 2500 mg powder as follows:
- Within the first hour after the first dose of the alternative drug, reduce the ESMOLOL HYDROCHLORIDE 2500 mg powder infusion rate by one-half (50%).
- After administration of the second dose of the other alternative drug, monitor the patient’s response and if satisfactory control is maintained for the first hour, discontinue the ESMOLOL HYDROCHLORIDE 2500 mg powder infusion.
Additional dosing information: as the desired therapeutic effect or a safety endpoint (e.g. lowered blood pressure) is approached, omit the loading dose and reduce the incremental infusion to 12.5–25 micrograms/kg/min. Also, if desired, increase the interval between titration steps from five to ten minutes.
ESMOLOL HYDROCHLORIDE 2500 mg powder should be discontinued when heart rate or blood pressure rapidly approach or exceed a safety limit, and then restarted without a loading infusion at a lower dose after the heart rate or blood pressure has returned to an acceptable level.
Special populations
Elderly
The elderly should be treated with caution, starting with a lower dosage. Special studies in the elderly have not been conducted. However, analysis of data of 252 patients over 65 years indicated that no variations in pharmacodynamic effects occurred as compared with data of patients under 65.
Patients with kidney insufficiency
In patients with renal insufficiency caution is needed when ESMOLOL HYDROCHLORIDE 2500 mg powder is administered by infusion, since the acid metabolite of ESMOLOL HYDROCHLORIDE 2500 mg powder is excreted through the kidneys. Excretion of the acid metabolite is significantly decreased in patients with renal disease, with the elimination half-life increased to about tenfold that of normals, and plasma levels considerably elevated.
Patients with liver insufficiency
In case of liver insufficiency no special precautions are necessary since the esterases in the red blood cells have a main role in the ESMOLOL HYDROCHLORIDE 2500 mg powder metabolism.
Paediatric population (age under 18 years):
The safety and efficacy of ESMOLOL HYDROCHLORIDE 2500 mg powder in children aged up to 18 years have not yet been established. Therefore, ESMOLOL HYDROCHLORIDE 2500 mg powder is not indicated for use in the paediatric population (see section 4.1).
Currently available data are described in section 5.1 and 5.2 but no recommendation on a posology can be made.
Method of Administration
The powder must be reconstituted/diluted before use. The reconstituted/diluted powder can be administered in two different concentrations in two different volumes:
- The standard concentration is 10 mg/ml, using a final volume of 250 ml
- In some cases where a lower volume is considered necessary, a higher concentration (50 mg/ml) can be prepared by diluting the powder in a final volume of 50 ml and administered with a PERFUSOR/MOTOR PUMP. There is limited clinical experience with the use of this higher concentration. This higher concentration should be infused only through a large vein or a central catheter using a perfusor pump (see section 4.4).
See section 6.6 for method of preparation.
INFUSION RATE CONVERSION TABLES (microgram/kg/min → ml/min) for a diluted solution for infusion (10 mg/ml) administered through STANDARD INFUSION
Conversion table: microgram/kg/min → ml/min (esmolol diluted to 10 mg/ml strength)
| 500 μg/kg/min | 50 μg/kg/min | 100 μg/kg/min | 150 μg/kg/min | 200 μg/kg/min | 250 μg/kg/min | 300 μg/kg/min | |
|---|---|---|---|---|---|---|---|
| 1 minute only | |||||||
| kg | ml/min | ml/min | ml/min | ml/min | ml/min | ml/min | ml/min |
| 40 | 2 | 0,2 | 0,4 | 0,6 | 0,8 | 1 | 1,2 |
| 45 | 2,25 | 0,225 | 0,45 | 0,675 | 0,9 | 1,125 | 1,35 |
| 50 | 2,5 | 0,25 | 0,5 | 0,75 | 1 | 1,25 | 1,5 |
| 55 | 2,75 | 0,275 | 0,55 | 0,825 | 1,1 | 1,375 | 1,65 |
| 60 | 3 | 0,3 | 0,6 | 0,9 | 1,2 | 1,5 | 1,8 |
| 65 | 3,25 | 0,325 | 0,65 | 0,975 | 1,3 | 1,625 | 1,95 |
| 70 | 3,5 | 0,35 | 0,7 | 1,05 | 1,4 | 1,75 | 2,1 |
| 75 | 3,75 | 0,375 | 0,75 | 1,125 | 1,5 | 1,875 | 2,25 |
| 80 | 4 | 0,4 | 0,8 | 1,2 | 1,6 | 2 | 2,4 |
| 85 | 4,25 | 0,425 | 0,85 | 1,275 | 1,7 | 2,125 | 2,55 |
| 90 | 4,5 | 0,45 | 0,9 | 1,35 | 1,8 | 2,25 | 2,7 |
| 95 | 4,75 | 0,475 | 0,95 | 1,425 | 1,9 | 2,375 | 2,85 |
| 100 | 5 | 0,5 | 1 | 1,5 | 2 | 2,5 | 3 |
| 105 | 5,25 | 0,525 | 1,05 | 1,575 | 2,1 | 2,625 | 3,15 |
| 110 | 5,5 | 0,55 | 1,1 | 1,65 | 2,2 | 2,75 | 3,3 |
| 115 | 5,75 | 0,575 | 1,15 | 1,725 | 2,3 | 2,875 | 3,45 |
| 120 | 6 | 0,6 | 1,2 | 1,8 | 2,4 | 3 | 3,6 |
Conversion table: microgram/kg/min → ml/hour (esmolol diluted to 10 mg/ml strength)
| 500 μg/kg/min | 50 μg/kg/min | 100 μg/kg/min | 150 μg/kg/min | 200 μg/kg/min | 250 μg/kg/min | 300 μg/kg/min | |
|---|---|---|---|---|---|---|---|
| 1 minute only | |||||||
| kg | ml/min | ml/min | ml/min | ml/min | ml/min | ml/min | ml/min |
| 40 | 120 | 12 | 24 | 36 | 48 | 60 | 72 |
| 45 | 135 | 13,5 | 27 | 40,5 | 54 | 67,5 | 81 |
| 50 | 150 | 15 | 30 | 45 | 60 | 75 | 90 |
| 55 | 165 | 16,5 | 33 | 49,5 | 66 | 82,5 | 99 |
| 60 | 180 | 18 | 36 | 54 | 72 | 90 | 108 |
| 65 | 195 | 19,5 | 39 | 58,5 | 78 | 97,5 | 117 |
| 70 | 210 | 21 | 42 | 63 | 84 | 105 | 126 |
| 75 | 225 | 22,5 | 45 | 67,5 | 90 | 112,5 | 135 |
| 80 | 240 | 24 | 48 | 72 | 96 | 120 | 144 |
| 85 | 255 | 25,5 | 51 | 76,5 | 102 | 127,5 | 153 |
| 90 | 270 | 27 | 54 | 81 | 108 | 135 | 162 |
| 95 | 285 | 28,5 | 57 | 85,5 | 114 | 142,5 | 171 |
| 100 | 300 | 30 | 60 | 90 | 120 | 150 | 180 |
| 105 | 315 | 31,5 | 63 | 94,5 | 126 | 157,5 | 189 |
| 110 | 330 | 33 | 66 | 99 | 132 | 165 | 198 |
| 115 | 345 | 34,5 | 69 | 103,5 | 138 | 172,5 | 207 |
| 120 | 360 | 36 | 72 | 108 | 144 | 180 | 216 |
INFUSION RATE CONVERSION TABLES (microgram/kg/min → ml/min) for a concentrated solution for infusion (50 mg/ml) administered with a PERFUSOR/MOTOR PUMP
Conversion table: microgram/kg/min → ml/min (esmolol diluted to 50 mg/ml strength)
| 500 μg/kg/min | 50 μg/kg/min | 100 μg/kg/min | 150 μg/kg/min | 200 μg/kg/min | 250 μg/kg/min | 300 μg/kg/min | |
|---|---|---|---|---|---|---|---|
| 1 minute only | |||||||
| kg | ml/min | ml/min | ml/min | ml/min | ml/min | ml/min | ml/min |
| 40 | 0,4 | 0,04 | 0,08 | 0,12 | 0,16 | 0,2 | 0,24 |
| 45 | 0,45 | 0,045 | 0,09 | 0,135 | 0,18 | 0,225 | 0,27 |
| 50 | 0,5 | 0,05 | 0,1 | 0,15 | 0,2 | 0,25 | 0,3 |
| 55 | 0,55 | 0,055 | 0,11 | 0,165 | 0,22 | 0,275 | 0,33 |
| 60 | 0,6 | 0,06 | 0,12 | 0,18 | 0,24 | 0,3 | 0,36 |
| 65 | 0,65 | 0,065 | 0,13 | 0,195 | 0,26 | 0,325 | 0,39 |
| 70 | 0,7 | 0,07 | 0,14 | 0,21 | 0,28 | 0,35 | 0,42 |
| 75 | 0,75 | 0,075 | 0,15 | 0,225 | 0,3 | 0,375 | 0,45 |
| 80 | 0,8 | 0,08 | 0,16 | 0,24 | 0,32 | 0,4 | 0,48 |
| 85 | 0,85 | 0,085 | 0,17 | 0,255 | 0,34 | 0,425 | 0,51 |
| 90 | 0,9 | 0,09 | 0,18 | 0,27 | 0,36 | 0,45 | 0,54 |
| 95 | 0,95 | 0,095 | 0,19 | 0,285 | 0,38 | 0,475 | 0,57 |
| 100 | 1 | 0,1 | 0,2 | 0,3 | 0,4 | 0,5 | 0,6 |
| 105 | 1,05 | 0,105 | 0,21 | 0,315 | 0,42 | 0,525 | 0,63 |
| 110 | 1,1 | 0,11 | 0,22 | 0,33 | 0,44 | 0,55 | 0,66 |
| 115 | 1,15 | 0,115 | 0,23 | 0,345 | 0,46 | 0,575 | 0,69 |
| 120 | 1,2 | 0,12 | 0,24 | 0,36 | 0,48 | 0,6 | 0,72 |
Conversion table: microgram/kg/min ml/hour (esmolol diluted to 50 mg/ml strength)
| 500 μg/kg/min | 50 μg/kg/min | 100 μg/kg/min | 150 μg/kg/min | 200 μg/kg/min | 250 μg/kg/min | 300 μg/kg/min | |
|---|---|---|---|---|---|---|---|
| 1 minute only | |||||||
| kg | ml/min | ml/min | ml/min | ml/min | ml/min | ml/min | ml/min |
| 40 | 24 | 2,4 | 4,8 | 7,2 | 9,6 | 12 | 14,4 |
| 45 | 27 | 2,7 | 5,4 | 8,1 | 10,8 | 13,5 | 16,2 |
| 50 | 30 | 3 | 6 | 9 | 12 | 15 | 18 |
| 55 | 33 | 3,3 | 6,6 | 9,9 | 13,2 | 16,5 | 19,8 |
| 60 | 36 | 3,6 | 7,2 | 10,8 | 14,4 | 18 | 21,6 |
| 65 | 39 | 3,9 | 7,8 | 11,7 | 15,6 | 19,5 | 23,4 |
| 70 | 42 | 4,2 | 8,4 | 12,6 | 16,8 | 21 | 25,2 |
| 75 | 45 | 4,5 | 9 | 13,5 | 18 | 22,5 | 27 |
| 80 | 48 | 4,8 | 9,6 | 14,4 | 19,2 | 24 | 28,8 |
| 85 | 51 | 5,1 | 10,2 | 15,3 | 20,4 | 25,5 | 30,6 |
| 90 | 54 | 5,4 | 10,8 | 16,2 | 21,6 | 27 | 32,4 |
| 95 | 57 | 5,7 | 11,4 | 17,1 | 22,8 | 28,5 | 34,2 |
| 100 | 60 | 6 | 12 | 18 | 24 | 30 | 36 |
| 105 | 63 | 6,3 | 12,6 | 18,9 | 25,2 | 31,5 | 37,8 |
| 110 | 66 | 6,6 | 13,2 | 19,8 | 26,4 | 33 | 39,6 |
| 115 | 69 | 6,9 | 13,8 | 20,7 | 27,6 | 34,5 | 41,4 |
| 120 | 72 | 7,2 | 14,4 | 21,6 | 28,8 | 36 | 43,2 |
Overdose
Cases of massive accidental overdoses with concentrated solutions of ESMOLOL HYDROCHLORIDE 2500 mg powder have occurred. Some of these overdoses have been fatal while others resulted in permanent disability. Loading doses in the range of 625 mg to 2.5 g (12.5-50 mg/kg) have been fatal.
Symptoms
In case of overdose the following symptoms can occur: severe hypotension, sinus bradycardia, atrioventricular block, heart insufficiency, cardiogenic shock, cardiac arrest, bronchospasm, respiratory insufficiency, loss of consciousness to coma, convulsions, nausea, vomiting, hypoglycemia and hyperkalemia.
Treatment
Because of the short elimination half-life of ESMOLOL HYDROCHLORIDE 2500 mg powder powder for concentrate for solution for infusion (approximately 9 minutes), the first step in the management of toxicity should be to discontinue the administration of the drug. The time taken for symptoms to disappear following overdosing will depend on the amount of ESMOLOL HYDROCHLORIDE 2500 mg powder administered. This may take longer than the 30 minutes seen with discontinuation at therapeutic dose levels of ESMOLOL HYDROCHLORIDE 2500 mg powder. Artificial respiration may be necessary. Based on the observed clinical effects, the following general measures should also be considered:
Bradycardia: atropine or another anticholinergic drug should be given i.v. When the bradycardia cannot be treated sufficiently a pacemaker may be necessary.
Bronchospasm: nebulised beta-2-sympathomimetics should be given. If this is not sufficient intravenous beta-2-sympathomimetics or aminophylline can be considered.
Symptomatic hypotension: fluids and/or pressor agents should be given i.v.
Cardiovascular depression or cardiac shock: diuretics or sympathomimetics can be administered. The dose of sympathomimetics (depending on the symptoms: dobutamine, dopamine, noradrenaline, isoprenaline, etc.) depends on the therapeutic effect.
In case further treatment is necessary, the following agents can be given i.v.: based on the clinical situation and judgment of the treating healthcare professional:
- Atropine: 0.5-2 mg
- inotropic agents
- calcium ions
Shelf life
60 months.
The in-use storage condition is 25°C.
The opened, reconstituted and diluted product is physicochemically stabile during 24 hours at 25°C. From microbiological point of view the product must be used immediately after opening and dilution. In case this is not done, the user is responsible for use and administration. Normally, the period of use is not more than 24 hours at 2-8°C, unless opening, reconstitution/dilution has taken place in controlled and validated aseptic conditions.
Special precautions for storage
This medicinal product does not require any special storage conditions. For storage conditions of the reconstituted solution see section 6.3.
Nature and contents of container
A clear, colourless, 50 ml glass vial with a bromobutyl rubber stopper and a flip off seal containing 2500 mg powder for concentrate for solution for infusion. The vial is packed in an outer cardboard carton.
Pack size: 1 vial per carton.
Special precautions for disposal and other handling
ESMOCARD LYO 2500 mg powder for concentrate for solution for infusion MUST NOT BE ADMINISTERED WITHOUT RECONSTITUTION/DILUTION.
The powder must be reconstituted/diluted before use. The reconstituted/diluted powder can be administered in two different concentrations in two different volumes (see section 4.2):
1. The powder can be administered as a diluted solution for infusion (10 mg/ml) with a volume of 250 ml through STANDARD INFUSION
OR
2. The powder can be administered as a concentrated solution for infusion (50 mg/ml) with a volume of 50 ml with a PERFUSOR/MOTOR PUMP. There is limited clinical experience with the use of this higher concentration. This higher concentration should be infused only through a large vein or a central catheter using a perfusor pump (see section 4.4).
INSTRUCTION FOR USE for a diluted solution for infusion (10 mg/ml) administered through STANDARD INFUSION
| Presentation | Volume of diluent to be added | Final concentration of the reconstituted /diluted solution | Final volume of the reconstituted /diluted solution | Administration |
|---|---|---|---|---|
| 2500 mg Esmolol powder | Step 1: Reconstitute one vial with 50ml of one of below mentioned solutions. Step 2: Dilute immediately the reconstituted content of the vial (50 ml) to 250 ml with one of below mentioned solutions. | 10 mg/ml | 250 ml | Standard infusion with a volume of 250 ml |
INSTRUCTION FOR USE for a concentrated solution for infusion (50 mg/ml) administered with a PERFUSOR/MOTOR PUMP
| Presentation | Volume of diluent to be added | Final concentration of the reconstituted solution | Final volume of the reconstituted solution | Administration |
|---|---|---|---|---|
| 2500 mg Esmolol powder | Reconstitute one vial with 50ml of one of below mentioned solutions. No further dilution is necessary. | 50 mg/ml | 50 ml | Use a perfusor/motor pump which takes syringes with a 50 ml volume |
Appropriate solutions for reconstitution and dilution are
NaCl 9 mg / ml (0.9%) solution
Glucose 50 mg / ml (5%) solution
Glucose 50 mg / ml (5%) in Ringer’s solution
Glucose 50 mg / ml (5%) in NaCl 9 mg / ml (0.9%) solution
Glucose 50 mg / ml (5%) in Ringer-lactate solution
Ringer-lactate solution
Diluents for the final solution for infusion are commonly used for intravenously administered liquids, in glass as well as in PVC bottles.
The white to almost white lyophilised powder will dissolve completely after reconstitution. Mix gently until a clear solution is obtained.
Reconstituted solutions should be visually examined for particulate matter and discoloration. Only a clear and colourless solution should be used.
The opened, reconstituted and diluted product is physicochemically stable during 24 hours at 25°C. From microbiological point of view the product must be used immediately. In case this is not done, the user is responsible for use and administration. Normally, the period of use is not more than 24 hours at 2-8°C, unless opening, reconstitution/dilution has taken place in controlled and validated aseptic conditions.
Any unused solution and the containers should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.