ESTRACE Tablet Ref.[10807] Active ingredients: Estradiol
Source: FDA, National Drug Code (US) Revision Year: 2016
Product description
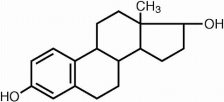
ESTRACE (estradiol tablets USP) for oral administration contains 0.5, 1 or 2 mg of micronized estradiol, USP per tablet. Estradiol, USP (17ß-estradiol) is a white, crystalline solid, chemically described as estra-1,3,5,(10)-triene-3, 17ß-diol. The structural formula is:
C18H24O2 M.W. 272.38
Inactive Ingredients: Colloidal silicon dioxide, corn starch, dibasic calcium phosphate, lactose monohydrate, magnesium stearate, and sodium starch glycolate. In addition, the 1 mg also contains FD&C blue no. 1 aluminum lake and D&C red no. 27 aluminum lake. The 2 mg also contains FD&C blue no. 1 aluminum lake and FD&C yellow no. 5 (tartrazine) aluminum lake.
| How Supplied |
|---|
|
ESTRACE (estradiol tablets USP) are available as: 0.5 mg: White to off-white, oval, flat-faced, beveled-edge, scored tablet. Debossed with 720/½ on the scored side and WC on the other side. Available in bottles of: 100 Tablets NDC 0430-0720-24 1 mg: Light purple, oval, flat-faced, beveled-edge, scored tablet. Debossed with 721/1 on the scored side and WC on the other side. Available in bottles of: 100 Tablets NDC 0430-0721-24 2 mg: Green, oval, flat-faced, beveled-edge, scored tablet. Debossed with 722/2 on the scored side and WC on the other side. Available in bottles of: 100 Tablets NDC 0430-0722-24 Mfd by: Teva Pharmaceuticals USA, Inc., North Wales, PA 19454 Marketed by: Allergan USA, Inc., Irvine, CA 92612 |
Drugs
| Drug | Countries | |
|---|---|---|
| ESTRACE | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.