Source: FDA, National Drug Code (US) Revision Year: 2020
Crisaborole is a phosphodiesterase 4 (PDE-4) inhibitor. PDE-4 inhibition results in increased intracellular cyclic adenosine monophosphate (cAMP) levels. The specific mechanism(s) by which crisaborole exerts its therapeutic action for the treatment of atopic dermatitis is not well defined.
At therapeutic doses, EUCRISA ointment is not expected to prolong QTc to any clinically relevant extent.
The PK of EUCRISA were investigated in 33 pediatric subjects 2 to 17 years of age with mild to moderate atopic dermatitis and a mean ± SD body surface area (BSA) involvement of 49 ± 20% (range 27% to 92%). In this study, subjects applied approximately 3 mg/cm2 of EUCRISA ointment (dose range was approximately 6 g to 30 g per application) twice daily for 8 days.
Plasma concentrations were quantifiable in all the subjects. The mean ± SD maximum plasma concentration (Cmax) and area under the concentration time curve from 0 to 12 hours post dose (AUC0-12) for crisaborole on Day 8 were 127 ± 196 ng/mL and 949 ± 1240 ng h/mL, respectively. Systemic concentrations of crisaborole were at steady state by Day 8. Based on the ratios of AUC0-12 between Day 8 and Day 1, the mean accumulation factor for crisaborole was 1.9.
The PK of EUCRISA were investigated in 13 subjects 4 months to less than 24 months of age. The mean ± SD Cmax and AUC0-12 for crisaborole were 188 ± 100 ng/mL and 1164 ± 550 ng∙h/mL, respectively.
Based on an in vitro study, crisaborole is 97% bound to human plasma proteins.
Crisaborole is substantially metabolized into inactive metabolites. The major metabolite 5-(4-cyanophenoxy)-2-hydroxyl benzylalcohol (metabolite 1), is formed via hydrolysis; this metabolite is further metabolized into downstream metabolites, among which 5-(4-cyanophenoxy)-2-hydroxyl benzoic acid (metabolite 2), formed via oxidation, is also a major metabolite.
PK of metabolites 1 and 2 were assessed in the PK study described above and the systemic concentrations were at or near steady state by Day 8. Based on the ratios of AUC0-12 between Day 8 and Day 1, the mean accumulation factors for metabolites 1 and 2 were 1.7 and 6.3, respectively.
Renal excretion of metabolites is the major route of elimination.
In vitro studies using human liver microsomes indicated that under the conditions of clinical use, crisaborole and metabolite 1 are not expected to inhibit cytochrome P450 (CYP) 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, and 3A4.
In vitro human liver microsomes studies for metabolite 2 showed that it did not inhibit activities of CYP2C19, 2D6, and 3A4; was a weak inhibitor of CYP1A2 and 2B6; and a moderate inhibitor of CYP2C8 and 2C9. The most sensitive enzyme, CYP2C9, was further investigated in a clinical trial using warfarin as a CYP2C9 substrate. The results of this study showed no drug interaction potential.
In vitro studies in human hepatocytes showed that under the conditions of clinical use, crisaborole and metabolites 1 and 2 are not expected to induce CYP enzymes.
In vitro studies showed that crisaborole and metabolite 1 did not inhibit the activities of uridine diphosphate (UDP)-glucuronosyltransferase (UGT) 1A1, 1A4, 1A6, 1A9, 2B7, and 2B15. Metabolite 2 did not inhibit UGT1A4, 1A6, 2B7, and 2B15. Metabolite 2 showed weak inhibition of UGT1A1, however, no clinically significant drug interactions are expected between crisaborole (and its metabolites) and UGT1A1 substrates at therapeutic concentrations. Metabolite 2 showed moderate inhibition of UGT1A9 and may result in a moderate increase of the concentrations of sensitive UGT1A9 substrates.
In vitro studies indicate that under the condition of clinical use, crisaborole and metabolites 1 and 2 are not expected to cause clinically significant interactions with substrates of P-glycoprotein and organic anionic or cationic transporters. Crisaborole and metabolite 1 are not expected to inhibit breast cancer resistance protein (BCRP); metabolite 2 is expected to inhibit BCRP at therapeutic concentrations.
In an oral carcinogenicity study in Sprague-Dawley rats, oral doses of 30, 100, or 300 mg/kg/day crisaborole were administered to rats once daily. A crisaborole-related increased incidence of benign granular cell tumors in the uterus with cervix and vagina (combined) was noted in 300 mg/kg/day crisaborole treated female rats (2 times the MRHD on an AUC comparison basis). The clinical relevance of this finding is unknown.
In a dermal carcinogenicity study in CD-1 mice, topical doses of 2%, 5%, or 7% crisaborole ointment were administered once daily. No crisaborole-related neoplastic findings were noted at topical doses up to 7% crisaborole ointment (1 times the MRHD on an AUC comparison basis).
Crisaborole revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and human lymphocyte chromosomal aberration assay) and one in vivo genotoxicity test (rat micronucleus assay).
No effects on fertility were observed in male or female rats that were administered oral doses up to 600 mg/kg/day crisaborole (13 times the MRHD on an AUC comparison basis) prior to and during early pregnancy.
Two multicenter, randomized, double-blind, parallel-group, vehicle-controlled trials (Trials 1 and 2) treated a total of 1522 subjects 2 to 79 years of age (86.3% of subjects were 2 to 17 years of age) with a 5% to 95% treatable BSA. At baseline, 38.5% of the subjects had an Investigator’s Static Global Assessment [ISGA] score of 2 (mild), and 61.5% had an ISGA score of 3 (moderate), in the overall assessment of atopic dermatitis (erythema, induration/papulation, and oozing/crusting) on a severity scale of 0 to 4.
In both trials, subjects were randomized 2:1 to receive EUCRISA or vehicle applied twice daily for 28 days. The primary efficacy endpoint was the proportion of subjects at Day 29 who achieved success, defined as an ISGA grade of Clear (score of 0) or Almost Clear (score of 1) with a 2-grade or greater improvement from baseline, comparing EUCRISA-treated subjects to vehicle-treated subjects.
Efficacy results from the two trials are summarized in Table 2.
Table 2. Primary Efficacy Outcomes in Subjects with Mild to Moderate Atopic Dermatitis at Day 29:
| Trial 1 | Trial 2 | |||
|---|---|---|---|---|
| EUCRISA (N=503) | Vehicle (N=256) | EUCRISA (N=513) | Vehicle (N=250) | |
| Success in ISGA* | 32.8% | 25.4% | 31.4% | 18.0% |
* Defined as an ISGA score of Clear (0) or Almost Clear (1) with a 2-grade or greater improvement from baseline.
The success rates over time are presented in Figure 1.
Figure 1. Success in ISGA* Over Time in Subjects with Mild to Moderate Atopic Dermatitis:
| Trial 1 | Trial 2 |
|---|---|
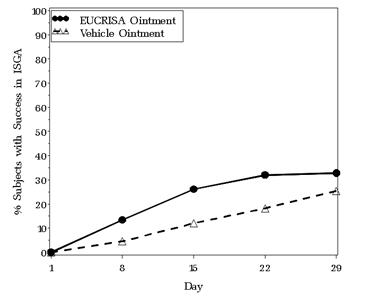 | 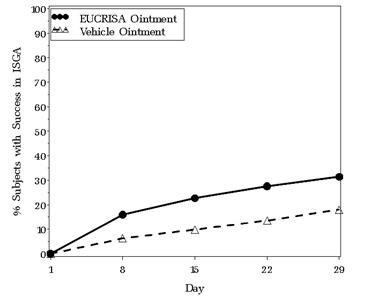 |
* Success is defined as an ISGA score of Clear (0) or Almost Clear (1) with a 2-grade or greater improvement from baseline.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.