EVENITY Solution for injection Ref.[10194] Active ingredients: Romosozumab
Source: FDA, National Drug Code (US) Revision Year: 2020
1. Indications and Usage
1.1 Treatment of Postmenopausal Women with Osteoporosis at High Risk for Fracture
EVENITY is indicated for the treatment of osteoporosis in postmenopausal women at high risk for fracture, defined as a history of osteoporotic fracture, or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy.
1.2 Limitations of Use
The anabolic effect of EVENITY wanes after 12 monthly doses of therapy. Therefore, the duration of EVENITY use should be limited to 12 monthly doses. If osteoporosis therapy remains warranted, continued therapy with an anti-resorptive agent should be considered [see Dosage and Administration (2.2) and Clinical Studies (14.1)].
2. Dosage and Administration
2.1 Important Dosage and Administration Instructions
- Two separate syringes (and two separate subcutaneous injections) are needed to administer the total dose of 210 mg of EVENITY. Inject two 105 mg/1.17 mL prefilled syringes, one after the other.
- EVENITY should be administered by a healthcare provider.
2.2 Recommended Dosage
- The recommended dose of EVENITY is 210 mg administered subcutaneously in the abdomen, thigh or upper arm. Administer EVENITY once every month.
- The treatment duration for EVENITY is 12 monthly doses.
- Patients should be adequately supplemented with calcium and vitamin D during treatment with EVENITY [see Warnings and Precautions (5.3) and Clinical Studies (14.1)].
- If the EVENITY dose is missed, administer as soon as it can be rescheduled. Thereafter, EVENITY can be scheduled every month from the date of the last dose.
2.3 Preparation and Administration Instructions
Step 1: Prior to Administration:
- Remove two syringes from the carton.
- Visually inspect EVENITY for particles and discoloration prior to administration. EVENITY is a clear to opalescent, colorless to light yellow solution. Do not use if the solution is cloudy or discolored or contains particles.
- Do not use the syringe if
- any part appears cracked or broken
- the gray needle cap is missing or not securely attached
- the expiration date printed on the label has passed
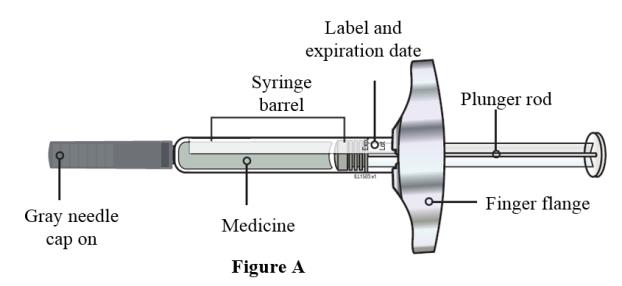
- Always hold the prefilled syringe by the syringe barrel to remove the syringe from the tray. See Figure A.
- Do not grasp the plunger rod.
- Do not grasp the gray needle cap.
- Do not remove the gray needle cap until you are ready to inject.
- Allow EVENITY to sit at room temperature for at least 30 minutes before injecting. Do not warm in any other way [see How Supplied/Storage and Handling (16)].
Step 2: Select the Injection Site and Prepare the Syringe:
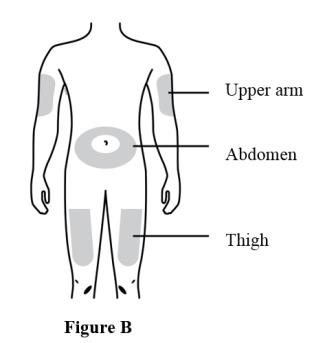
Prepare and clean two injection sites, one for each of the two injections. See Figure B.
The recommended subcutaneous injection sites include:
- The thigh
- Abdomen, except for a two-inch area right around the navel
- Outer area of upper arm
Clean the injection sites with alcohol wipes. Let the skin dry.
- Choose a different site each time you give an injection. If you want to use the same injection site, make sure it is not the same spot on the injection site you used for a previous injection.
- Do not inject into areas where the skin is tender, bruised, red, or hard. Avoid injecting into areas with scars or stretch marks.
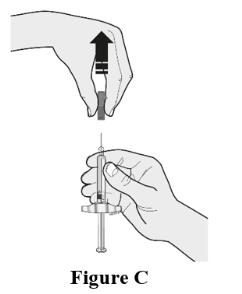
Choose the first syringe. Pull the gray needle cap straight off and away from your body when you are ready to inject. See Figure C.
- Do not put the gray needle cap back onto the syringe.
Step 3: Inject EVENITY
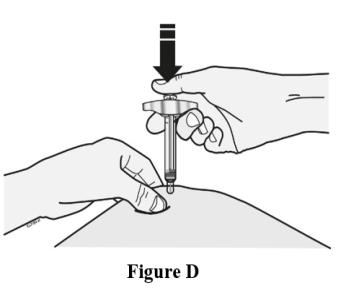
Insert needle and inject all the liquid subcutaneously. Do not administer into muscle or blood vessel. See Figure D.
When done, gently lift the syringe off of the skin.
Step 4: Syringe and Needle Cap Disposal
Immediately dispose of the syringe and needle cap in the nearest sharps container.
Important: Repeat all steps with the second syringe to inject the full dose.
16.2. Storage and Handling
- Refrigerate EVENITY at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze. Do not shake.
- If removed from the refrigerator, EVENITY can be kept at room temperature up to 25°C (77°F) in the original carton and must be used within 30 days. If not used within 30 days, discard EVENITY.
- Do not expose EVENITY to temperatures above 25°C (77°F).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.