EVKEEZA Concentrate for solution for infusion Ref.[27942] Active ingredients: Evinacumab
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Ultragenyx Germany GmbH, Rahel-Hirsch-Str. 10, 10557 Berlin, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other lipid modifying agents
ATC code: C10AX17
Mechanism of action
Evinacumab is a recombinant human monoclonal antibody, which specifically binds to and inhibits ANGPTL3. ANGPTL3 is a member of the angiopoietin-like protein family that is expressed primarily in the liver and plays a role in the regulation of lipid metabolism by inhibiting lipoprotein lipase (LPL) and endothelial lipase (EL).
Evinacumab blockade of ANGPTL3 lowers TG and HDL-C by releasing LPL and EL activities from ANGPTL3 inhibition, respectively. Evinacumab reduces LDL-C independent of the presence of LDL receptor (LDLR) by promoting very low-density lipoprotein (VLDL) processing and VLDL remnants clearance upstream of LDL formation through EL-dependent mechanism.
Clinical efficacy and safety
Homozygous familial hypercholesterolaemia (HoFH)
Study ELIPSE-HoFH
This was a multicentre, double-blind, randomised, placebo-controlled trial evaluating the efficacy and safety of evinacumab compared to placebo in 65 patients with HoFH. The trial consisted of a 24-week double-blind treatment period and a 24-week open-label treatment period. In the double-blind treatment period, 43 patients were randomised to receive evinacumab 15 mg/kg IV every 4 weeks and 22 patients to receive placebo. Patients were on a background of other lipid-lowering therapies (e.g. statins, ezetimibe, PCSK9 inhibitor antibodies, lomitapide, and lipoprotein apheresis). The diagnosis of HoFH was determined by genetic testing or by the presence of the following clinical criteria: history of an untreated TC >500 mg/dl (13 mmol/l) together with either xanthoma before 10 years of age or evidence of TC >250 mg/dl (6.47 mmol/l) in both parents. Patients regardless of mutation status were included in the trial. Patients were defined as having null/null or negative/negative variants if the variations resulted in little to no residual LDLR function; null/null variants were defined as having <15% LDLR function based on in vitro assays and negative/negative variants were defined as having premature termination codons, splice site variations, frame shifts, insertion/deletions or copy number variations. In this trial, 32.3% (21 of 65) of patients had null/null variants and 18.5% (12 of 65) of patients had negative/negative variants.
The mean LDL-C at baseline was 255.1 mg/dl (6.61 mmol/l) and in the subset of patients with null/null variants was 311.5 mg/dl (8.07 mmol/l) and with negative/negative variants was 289.4 mg/dl (7.50 mmol/l). At baseline, 93.8% of patients were on statins, 75.4% on ezetimibe, 76.9% on a PCSK9 inhibitor antibodies, 21.5% on lomitapide, and 33.8% were receiving lipoprotein apheresis. The mean age at baseline was 42 years (range 12 to 75) with 12.3% ≥65 years old; 53.8% women, 73.8% White, 15.4% Asian, 3.1% Black and 7.7% Other or not reported.
The primary efficacy end-point was percent change in LDL-C from baseline to week 24. At week 24, the LS mean treatment difference between evinacumab and placebo in mean percent change in LDL-C from baseline, was -49.0% (95% CI: -65.0% to -33.1%; p <0.0001). For efficacy results see Table 2.
Table 2. Effect of evinacumab on lipid parameters in patients with HoFH in study ELIPSE-HoFH:
| Baseline (mean), mmol/l (N=65) | LS mean percent change or change from baseline at week 24 | Difference from placebo (95% CI) | P-value | ||
|---|---|---|---|---|---|
| evinacumab (N=43) | placebo (N=22) | ||||
| LDL-C (percent change) | 6.6 | -47.1% | +1.9% | -49% (-65.0 to -33.1) | <0.0001 |
| LDL-C (absolute change) (mmol/l) | 6.6 | -3.5 | -0.1 | -3.4 (-4.5 to -2.3) | <0.0001 |
| ApoB (g/l) | 1.7 | -41.4% | -4.5% | -36.9% (-48.6 to -25.2) | <0.0001 |
| Non-HDL-C | 7.2 | -49.7% | +2.0% | -51.7% (-64.8 to -38.5) | <0.0001 |
| TC | 8.3 | -47.4% | +1.0% | -48.4% (-58.7 to -38.1) | <0.0001 |
| TG | 1.4 | -55.0% | -4.6% | -50.4% (-65.6 to -35.2) | <0.0001a |
| HDL-Cb | 1.2 | -29.6% | +0.8% | - | - |
a nominal p-value since TG is not a key secondary endpoint
b Mean percent change at week 24 results are presented based on the actual treatment received in safety population (evinacumab, n=44; placebo, n=20); there is no formal statistical testing in safety population
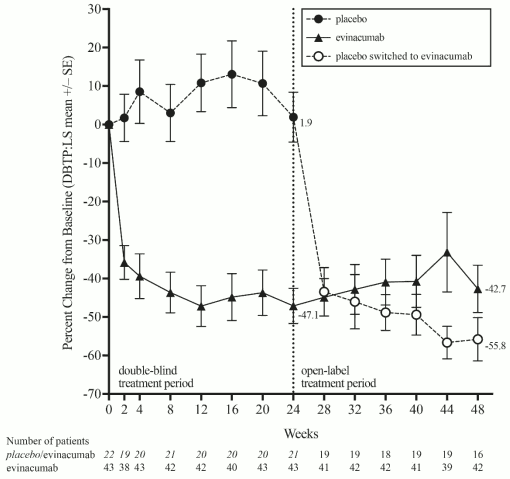
After the double-blind treatment period, 64 of the 65 randomised patients who entered the open-label treatment period received evinacumab. The mean percent change in LDL-C from baseline to week 48 ranged from -42.7% to -55.8%. Figure 1 shows the LDL-C mean percent change from baseline for the double-blind and observed mean percent change for the open-label treatment periods across patients who were on evinacumab or placebo during the double-blind treatment period.
Figure 1. Calculated LDL-C LS mean percent change from baseline over time through week 24, and observed mean percent change from week 28 through week 48 in study ELIPSE-HoFH:
At Week 24, the observed reduction in LDL-C with evinacumab was similar across predefined subgroups, including age, sex, null/null or negative/negative variants, concomitant treatment with lipoprotein apheresis, and concomitant background lipid-lowering medications (statins, ezetimibe, PCSK9 inhibitor antibodies, and lomitapide). The effect of evinacumab on cardiovascular morbidity and mortality has not been determined.
Study ELIPSE-OLE
In an ongoing multicentre, open-label extension study in 116 patients with HoFH, data available from 86 patients at 24 weeks showed a 43.6% decrease in LDL-C following evinacumab treatment 15 mg/kg IV every 4 weeks on top of other lipid-lowering therapies (e.g., statins, ezetimibe, PCSK9 inhibitor antibodies, lomitapide, and lipoprotein apheresis). Reductions from baseline in LDL-C were consistent at 48 and 96 weeks; the mean percent change from baseline in calculated LDL-C at 48 weeks (n=95) was -43.9% and at 96 weeks (n=63) was -37.2%. Patients regardless of mutation status were included in the trial, including patients with null/null or negative/negative variants.
Paediatric population
ELIPSE-HoFH
In ELIPSE-HoFH, 1 adolescent patient received 15 mg/kg IV of evinacumab every 4 weeks and 1 adolescent patient received placebo, as an adjunct to other lipid-lowering therapies (e.g., statins, ezetimibe, PCSK9 inhibitor antibodies and lipoprotein apheresis). Both adolescent patients had null/null variants in the LDLR. At Week 24, the percent change in LDL-C with evinacumab was -73.3% and with placebo +60%.
ELIPSE-OLE
In ELIPSE-OLE, 14 adolescent patients received 15 mg/kg IV of evinacumab every 4 weeks as an adjunct to other lipid-lowering therapies (e.g., statins, ezetimibe, PCSK9 inhibitor antibodies and lipoprotein apheresis). Two patients entered after completing the ELIPSE-HoFH study and 12 patients were evinacumab-naïve. The mean baseline LDL-C in these adolescent patients was 300.4 mg/dl (7.88 mmol). The mean age was 14.4 years (range: 12 to 17 years), with 64.3% males and 35.7% females. At baseline, all patients were on a statin, 71.4% on ezetimibe, 42.9% on PCSK9 inhibitor, and 64.3% were receiving lipoprotein apheresis. Four (28.6%) patients had null/null variants and 4 (28.6%) patients had negative/negative variants for LDLR mutations. At Week 24, the percent change in LDL-C with evinacumab was -55.4% (n=12).
Study R1500-CL-17100
This is an ongoing multicenter, three-part, single-arm, open-label study evaluating the efficacy, safety, and tolerability of evinacumab in paediatric patients aged ≥ 5 to 11 years with HoFH. The study includes three parts: Part A, Part B, and Part C. Part A was a single-dose, open-label study to assess the safety, PK, and PD of evinacumab 15 mg/kg IV in 6 patients with HoFH followed by a 16--week observational period to determine the dose for the rest of the study. Part B was a single-arm, 24--week, open-label treatment period evaluating the efficacy and safety of evinacumab 15 mg/kg IV every 4 weeks in 14 patients with HoFH. Part C is an extension study from Part A and Part B evaluating the long-term safety of evinacumab 15 mg/kg IV every 4 weeks in 20 patients with HoFH. It consists of a 48-week treatment period and a 24-week follow-up period (ongoing). Patients in Part C entered directly from Part A or Part B.
Patients were on any combination of lipid-lowering therapies, including maximally tolerated statins, ezetimibe, lomitapide, and lipoprotein apheresis.
The diagnosis of HoFH was determined by genetic testing or by the presence of the following clinical criteria: history of untreated total cholesterol (TC) >13 mmol/l (>500 mg/dl) and TG <7.8 mmol/l (<690 mg/dl) AND either tendinous xanthoma before 10 years of age or evidence of TC >6.47 mmol/l (>250 mg/dl) in both parents; LDL-C >3.36 mmol/l (>130 mg/dl); body weight ≥15 kg.
Overall, for patients in Part A and Part B, the mean LDL-C at baseline was 7.8 mmol/l (301.9 mg/dl). At baseline, 90% of patients were on statins, 95% were on ezetimibe, and 60% were receiving lipoprotein apheresis.
The mean age at baseline was 9.0 years (range ≥5 to <12); 40% males and 60% females; 70% White, 5% Black, 10% Asian, 5% American Indian or Alaska Native, and 10% Other. Mean body weight was 37.9 kg, and body mass index (BMI) was 18.8 kg/m².
In Part B, the primary efficacy endpoint was percent change in calculated LDL-C from baseline to week 24. At week 24, the mean percent change in calculated LDL-C from baseline was −48.3% (95% confidence interval: −68.8% to −27.8%). For efficacy results, see Table 3.
Table 3. Lipid parameters in paediatric patients (≥5 to 11 years) with HoFH on other lipid-lowering therapies at week 24:
| LDL-C | ApoB | Non-HDL-C | TC | Lp(a) | |
|---|---|---|---|---|---|
| Baseline (mean) (N=14) | 6.8 mmol/l (263.7 mg/dl) | 168.2 mg/dl (1.682 g/l) | 7.3 mmol/l (282.2 mg/dl) | 8.1 mmol/l (315.5 mg/dl) | 158.6 nmol/L |
| Percent change from baseline (95% CI) | -48.3 (-68.8 to -27.8) | -41.3 (-58.9 to -23.8) | -48.9 (-68.1 to -29.7) | -49.1 (-64.9 to -33.2) | -37.3 (-42.2 to -32.3) |
At week 24, the reduction in LDL-C with evinacumab was similar across baseline characteristics, including age, sex, limited LDL-R activity, concomitant treatment with lipoprotein apheresis, and concomitant background lipid-lowering medications (statins, ezetimibe, and lomitapide).
Other investigations
The efficacy of evinacumab for paediatric patients aged 6 months to less than 5 years has been predicted based on integrated PK/PD modelling and simulations (see section 5.2). Paediatric patients aged 6 months to less than 5 years receiving evinacumab 15 mg/kg every 4 weeks are predicted to experience a similar or higher magnitude of percent change in LDL-C at week 24 compared to adults while plateauing at higher absolute LDL-C concentrations at week 24.
In addition, data are available for 5 patients aged ≥1 to 5 years old with HoFH who received evinacumab via compassionate use. The prescribed dose was 15 mg/kg evinacumab every 4 weeks, the same as that used in older children and adults. Administration of evinacumab showed a clinically meaningful reduction of LDL-C consistent with that observed in patients ≥ 5 years old in clinical studies. The benefits included a reduction in LDL-C of 37.1% at week 90 in one of the patients in whom plasmapheresis frequency was reduced during the treatment period, and reductions of 43.1% at week 72, 66.3% at week 62, 77.3% at week 16, and 75.0% at week 12 respectively in the other patients. Xanthomas completely resolved in the patient in whom plasmapheresis frequency was reduced, after approximately 1 year of treatment with evinacumab.
This medicine has been authorised under ‘exceptional circumstances’. This means that due to the rarity of the disease it has not been possible to obtain complete information on this medicinal product. The European Medicines Agency will review any new information which may become available every year and this SmPC will be updated as necessary.
5.2. Pharmacokinetic properties
Absorption
Evinacumab is administered intravenously to patients with HoFH. Based on population PK modelling, at the end of infusion at steady-state, mean ± SD Cmax is 681 ± 185 mg/l in adult patients following a dose of 15 mg/kg every 4 weeks. The accumulation ratio is approximately 2. The mean ± SD steady-state trough concentration is 230 ± 81.3 mg/l in adult patients.
Distribution
The steady-state volume of distribution estimated by population PK analysis in a typical individual weighing 72 kg was approximately 4.9 L in adult patients, indicating that evinacumab is distributed primarily in the vascular system.
Biotransformation
The specific metabolism studies were not conducted because evinacumab is a protein. As a human monoclonal IgG4 antibody, evinacumab is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
Elimination
Evinacumab elimination is mediated by parallel linear and nonlinear pathways. At higher concentrations, evinacumab elimination is primarily through a non-saturable proteolytic pathway, while at lower concentrations, the non-linear saturable ANGPTL3 target-mediated elimination predominates. Elimination half-life is a function of evinacumab concentrations in serum and is not a constant.
After the last steady-state dose of 15 mg/kg IV every 4 weeks, the median time for evinacumab concentrations to decrease below the lower limit of detection (78 ng/ml) is 19 weeks.
Linearity/non-linearity
Due to nonlinear clearance, a slightly greater than dose proportional increase was observed, with a 4.3-fold increase in area under the concentration-time curve at steady-state (AUCtau.ss) for a 3-fold increase in dose from 5 mg/kg to 15 mg/kg IV every 4 weeks.
Pharmacokinetic/pharmacodynamic relationship(s)
The pharmacodynamic effect of evinacumab in lowering LDL-C is indirect, and mediated through the binding to ANGPTL3. Concentration of total ANGPTL3 increases from baseline upon administration of evinacumab and the increases plateau when target saturation is approached. When target is saturated, further increase in evinacumab concentrations is not expected to result in a further LDL-C reduction.
Special populations
A population PK analysis conducted on data from 183 healthy adult participants and 139 patients with HoFH, suggests that the following factors have no clinically significant effect on the exposure of evinacumab: age (5 to 75 years), gender, body weight (19.7 to 152 kg), race. Apheresis did not appear to substantially influence the pharmacokinetics of evinacumab.
Paediatric population
There were 14 patients aged 12 to 17 years with HoFH receiving evinacumab at 15 mg/kg IV every 4 weeks, steady-state trough and maximum concentrations were generally within the range of those in adult patients. The mean steady-state Cmax was 566 ± 206 mg/l in patients aged 12 to <18 years with HoFH.
For the 20 patients aged 5 to 11 years with HoFH receiving evinacumab at 15 mg/kg IV every 4 weeks, the mean (SD) steady-state trough evinacumab concentration based on population PK analyses was 160 ± 57.6 mg/l and the mean (SD) steady-state Cmax was 419 ± 99.4 mg/l in patients aged 5 to 11 years with HoFH.
The pharmacokinetics of evinacumab in paediatric patients less than 5 years of age with HoFH were predicted from a model-based extrapolation analysis. This analysis used population PK modelling and simulations based on previously observed data in older children, adolescents, and adults, together with assumptions on the biological development and pathophysiological circumstances in younger children with HoFH. The predicted mean steady-state trough concentrations and mean accumulation ratios in patients 6 months to less than 5 years were lower but within the ranges predicted for patients aged 5 years and older. The predicted mean steady-state maximum concentration was 499±185 mg/L for patients aged 6 months to less than 2 years and 513±179 mg/L for patients aged 2 to less than 5 years.
Renal impairment
Evinacumab is not expected to undergo significant renal elimination. Observed trough concentrations at steady-state were comparable between patients with mild or moderate renal impairment and patients with normal renal function. No data are available in patients with severe renal impairment.
Hepatic impairment
Evinacumab is not expected to undergo significant hepatic elimination. No data are available in patients with hepatic impairment.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology and repeated dose toxicity.
Carcinogenicity and mutagenicity
Carcinogenicity and genotoxicity studies have not been conducted with evinacumab. Monoclonal antibodies are not expected to alter DNA or chromosomes.
Reproductive toxicology
No effects on surrogate markers of fertility in male and female reproductive organs were observed in a 6-month chronic toxicology study with sexually mature cynomolgus monkeys. In animal reproduction studies, evinacumab was administered subcutaneously to pregnant rabbits every 3 days from gestation day 7 until gestation day 19 during organogenesis. Maternal toxicity (premature neonatal death, foetal loss and/or premature delivery) was observed at all doses and foetal findings (soft tissues and skeletal malformations) were observed at all but the lowest dose (1 mg/kg). Mean systemic exposure measured during the gestation period in rabbits was below that measured at maximum recommended human dose (MRHD) of 15 mg/kg every 4 weeks. Because the lipid profile of rabbits differs significantly from that of humans, particularly during pregnancy, the clinical relevance of these results is uncertain.
There were no effects on embryo-foetal development when rats were subcutaneously administered evinacumab every 3 days from gestation day 6 to gestation day 18 during organogenesis. Mean systemic exposure measured during the gestation period in rats was below that measured at MRHD of 15 mg/kg every 4 weeks.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.