EXONDYS 51 Solution for injection Ref.[10204] Active ingredients: Eteplirsen
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
EXONDYS 51 (eteplirsen) injection is a sterile, aqueous, preservative-free, concentrated solution for dilution prior to intravenous administration. EXONDYS 51 is clear and colorless, and may have some opalescence, and may contain trace amounts of small, white to off-white amorphous particles. EXONDYS 51 is supplied in single dose vials containing 100 mg or 500 mg eteplirsen (50 mg/mL). EXONDYS 51 is formulated as an isotonic, phosphate buffered saline solution with an osmolality of 260 to 320 mOsm and a pH of 7.5. Each milliliter of EXONDYS 51 contains 50 mg eteplirsen; 0.2 mg potassium chloride, 0.2 mg potassium phosphate monobasic, 8 mg sodium chloride, and 1.14 mg sodium phosphate dibasic, anhydrous, in water for injection. The product may contain hydrochloric acid or sodium hydroxide to adjust pH.
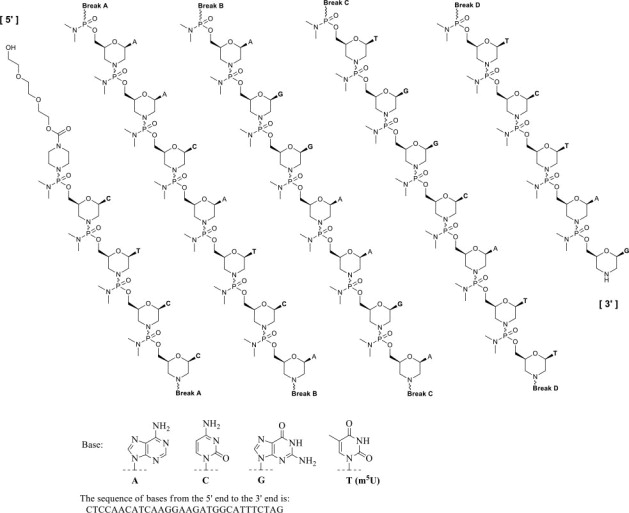
Eteplirsen is an antisense oligonucleotide of the phosphorodiamidate morpholino oligomer (PMO) subclass. PMOs are synthetic molecules in which the five-membered ribofuranosyl rings found in natural DNA and RNA are replaced by a six-membered morpholino ring. Each morpholino ring is linked through an uncharged phosphorodiamidate moiety rather than the negatively charged phosphate linkage that is present in natural DNA and RNA. Each phosphorodiamidate morpholino subunit contains one of the heterocyclic bases found in DNA (adenine, cytosine, guanine, or thymine). Eteplirsen contains 30 linked subunits. The molecular formula of eteplirsen is C364H569N177O122P30 and the molecular weight is 10305.7 daltons.
The structure and base sequence of eteplirsen are:
| Dosage Forms and Strengths |
|---|
|
EXONDYS 51 is a clear and colorless solution that may have some opalescence, and may contain trace amounts of small, white to off-white amorphous particles, and is available as follows:
|
| How Supplied |
|---|
|
EXONDYS 51 injection is supplied in single-dose vials. The solution is clear and colorless, and may have some opalescence, and may contain trace amounts of small, white to off-white amorphous particles.
|
Drugs
| Drug | Countries | |
|---|---|---|
| EXONDYS 51 | Israel, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.