EZETIMIBE Tablet Ref.[51696] Active ingredients: Ezetimibe
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
Ezetimibe, USP is a dietary cholesterol absorption inhibitor. The chemical name of ezetimibe is 1-(4-fluorophenyl)-3-( R )-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone. The empirical formula is C24H21F2NO3. Its molecular weight is 409.4 and its structural formula is:
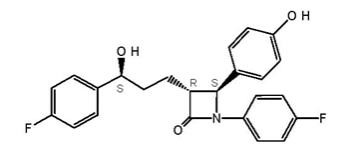
Ezetimibe, USP is a white, crystalline powder that is freely to very soluble in ethanol, methanol, and acetone and practically insoluble in water. Ezetimibe, USP has a melting point of about 163°C and is stable at ambient temperature. Ezetimibe, USP is available as a tablet for oral administration containing 10 mg of ezetimibe and the following inactive ingredients: croscarmellose sodium, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate.
| Dosage Forms and Strengths |
|---|
|
10-mg tablets are white to off-white, capsule-shaped tablets debossed with "713" on one side and plain on the other side. |
| How Supplied |
|---|
|
Ezetimibe tablets, USP 10 mg, are white to off white, capsule-shaped tablets debossed with "713" on one side and plain on the other side. They are supplied as follows: NDC 0591-3713-30 bottles of 30 Manufactured In India By: Watson Pharma Private Limited, Verna, Salcette Goa 403 722 INDIA Manufactured For: Teva Pharmaceuticals, Parsippany, NJ 07054 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.