FABHALTA Hard capsule Ref.[107283] Active ingredients: Iptacopan
Source: FDA, National Drug Code (US) Revision Year: 2025
12.1. Mechanism of Action
Iptacopan binds to Factor B of the alternative complement pathway and regulates the cleavage of C3, generation of downstream effectors, and the amplification of the terminal pathway.
In PNH, intravascular hemolysis (IVH) is mediated by the downstream membrane attack complex (MAC), while extravascular hemolysis (EVH) is facilitated by C3b opsonization. Iptacopan acts proximally in the alternative pathway of the complement cascade to control both C3b-mediated EVH and terminal complement-mediated IVH.
In IgAN, the deposition of galactose deficient IgA1 (Gd-IgA1) containing immune complexes in the kidney locally activates the alternative complement pathway which is thought to contribute to the pathogenesis of IgAN. By binding to Factor B, iptacopan inhibits the alternative pathway.
In C3G, overactivation of the alternative complement pathway leads to C3 cleavage within the glomeruli resulting in C3 deposition and inflammation, which are thought to contribute to the pathogenesis of C3G. By binding to Factor B, iptacopan inhibits the alternative pathway.
12.2. Pharmacodynamics
Inhibition of the alternative complement pathway biomarkers, in vitro alternative pathway assay and plasma Bb (fragment Bb of Factor B), started approximately 2 hours after a single iptacopan dose in healthy volunteers.
In PNH patients receiving concomitant anti-C5 treatment and FABHALTA 200 mg twice daily, the in vitro alternative pathway assay and plasma Bb decreased from baseline by 54.1% and 56.1%, respectively, on the first observation on Day 8. In treatment naïve PNH patients, these same biomarkers decreased from baseline by 78.4% and 58.9%, respectively, on the first observation after 4 weeks of treatment with FABHALTA 200 mg twice daily.
In PNH patients on concomitant anti-C5 treatment and FABHALTA 200 mg twice daily, the mean PNH red blood cell (RBC) clone size was 54.8% at baseline and increased to 89.2% after 13 weeks; the proportion of PNH Type II + III RBCs with C3 deposition was 12.4% at baseline and decreased to 0.2% after 13 weeks. In treatment naïve PNH patients, the mean PNH RBC clone size was 49.1% at baseline and increased to 91.1% after 12 weeks; there were negligible PNH Type II + III RBCs with C3 deposition in this population due to the predominance of IVH.
Iptacopan reduces serum LDH levels. In PNH patients previously treated with eculizumab, all patients treated with FABHALTA 200 mg twice daily achieved a reduction of LDH levels to <1.5 times upper limit of normal (ULN) at 13 weeks. In treatment naïve PNH patients, FABHALTA 200 mg twice daily reduced LDH by >60% compared to baseline after 12 weeks and maintained the effect through the end of the study at 2 years.
In IgAN patients receiving 200 mg twice daily, the in vitro alternative pathway assay, plasma Bb, plasma soluble C5b-9 (also known as MAC), and urine soluble C5b-9 decreased from baseline by 85.2%, 17.5%, 19.5% and 96.5%, respectively, on the first observation at Month 9.
In C3G patients receiving 200 mg twice daily, the geometric mean serum C3 at baseline was 23 mg/dL and increased to 80 mg/dL at Day 14 of FABHALTA treatment. Over this same period, the placebo group geometric mean serum C3 level decreased from 25 mg/dL to 24 mg/dL. At 6 months the mean glomerular C3 deposition score (0-12) decreased by 0.8 (95% CI: -0.3, 1.8) from a baseline of 9.2 with FABHALTA and increased by 1.1 (95% CI: 0.1, 2.1) from a baseline of 9.6 with placebo. Plasma soluble C5b-9 (also known as MAC) and urine soluble C5b-9 decreased from baseline by 67% and 88%, respectively, at Day 180 of treatment with FABHALTA 200 mg twice daily compared to a 3% decrease in plasma soluble C5b-9 and a 36% decrease in urine soluble C5b-9 in the placebo group.
Cardiac Electrophysiology
In a QTc clinical study in healthy volunteers, single supra-therapeutic iptacopan doses up to 1,200 mg (which provided greater than 4-fold peak concentration of the MRHD) showed no effect on cardiac repolarization or QT interval.
12.3. Pharmacokinetics
Absorption
Following oral administration, iptacopan reached peak plasma concentrations approximately 2 hours post dose. At the recommended dosing regimen of 200 mg twice daily, steady state is achieved in approximately 5 days with minor accumulation (1.4-fold).
Effect of Food
Based on a food-effect study in healthy volunteers, a high-fat meal did not affect the exposure of iptacopan to a clinically meaningful degree.
Distribution
Iptacopan showed concentration-dependent plasma protein binding due to binding to the target Factor B in the systemic circulation. Iptacopan was 75% to 93% protein bound in vitro at the relevant clinical plasma concentrations. After administration of iptacopan 200 mg twice daily, the apparent volume of distribution at steady state was approximately 288 L.
Elimination
The half-life (t1/2) of iptacopan at steady state is approximately 25 hours after administration of FABHALTA 200 mg twice daily. The apparent clearance of iptacopan at steady state is 8 L/h after administration of FABHALTA 200 mg twice daily.
Metabolism
Metabolism is a predominant elimination pathway for iptacopan with approximately 50% of the dose attributed to oxidative pathways. Metabolism of iptacopan includes N-dealkylation, O-deethylation, oxidation, and dehydrogenation, mostly driven by CYP2C8 (98%) with a small contribution from CYP2D6 (2%). Iptacopan undergoes Phase 2 metabolism through glucuronidation by UGT1A1, UGT1A3, and UGT1A8. In plasma, iptacopan was the major component, accounting for 83% of the drug-related species. Two acyl glucuronides were the only metabolites detected in plasma and were minor, accounting for 8% and 5% of the drug-related species. Iptacopan metabolites are not pharmacologically active.
Excretion
In a human study, following a single 100 mg oral dose of [14C]-iptacopan, mean total excretion of radioactivity (iptacopan and metabolites) was 72% in the feces and 25% in the urine, for a total mean excretion of >96% of the dose. Specifically, 18% of the dose was excreted as parent iptacopan in the urine, and 17% of the dose was excreted as parent iptacopan in feces.
Linearity / Non-linearity
At doses between 25 mg and 200 mg twice daily, iptacopan was overall less than dose proportional. However, oral doses of 100 mg and 200 mg were approximately dose proportional.
Specific Populations
A population pharmacokinetic (PK) analysis was conducted on iptacopan data from 234 patients. Age, body weight, race, and gender did not have a clinically significant effect on iptacopan PK.
Patients with Renal Impairment
There were no clinically significant differences in the exposure of FABHALTA between patients with an eGFR in the range of 25 to <90 mL/min compared to those with normal eGFR. No data are currently available in patients on dialysis.
Patients with Hepatic Impairment
In a study in subjects with normal hepatic function and patients with mild (Child-Pugh class A), moderate (Child-Pugh class B), or severe hepatic impairment (Child-Pugh class C), there was a negligible effect of hepatic impairment on the total (bound+unbound) exposure of iptacopan. However, unbound iptacopan AUCinf increased by 1.5, 1.6, and 3.7-fold in patients with mild, moderate, and severe hepatic impairment, respectively, compared to subjects with normal hepatic function.
Drug Interaction Studies
Based on a clinical drug interaction study in healthy volunteers, iptacopan exposure did not change to a clinically relevant degree when coadministered with clopidogrel (a moderate CYP2C8 inhibitor) or cyclosporine (a P-gp, BCRP, and OATP 1B1/1B3 inhibitor). The exposure of digoxin (a P-gp substrate) and rosuvastatin (an OATP substrate) did not change to a clinically relevant degree when coadministered with iptacopan.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Iptacopan was not genotoxic or mutagenic in a battery of in vitro and in vivo assays.
Carcinogenicity studies conducted with oral administration of iptacopan in RasH2 transgenic mice with doses up to 1,000 mg/kg/day for 6 months and in rats with doses up to 750 mg/kg/day for 2 years did not identify any carcinogenic potential. The highest exposure to iptacopan in rats corresponds to ~9-times the MRHD based on AUC.
In a fertility study in male rats, iptacopan did not adversely impact fertility up to the highest tested dose of 750 mg/kg/day, which corresponds to 4-times the MRHD based on AUC. Reversible effects on the male reproductive system (testicular tubular degeneration and cellular debris in epididymis) were observed in repeat-dose toxicity studies with oral administration in dogs at doses ≥2-times the MRHD based on AUC, with no clear effects on sperm numbers, morphology or motility. In a fertility and early embryonic developmental study in female rats, oral administration of iptacopan caused increased pre- and post-implantation losses when given at the highest dose of 1,000 mg/kg/day orally, which corresponds to ~11-times the MRHD based on AUC.
14. Clinical Studies
14.1 Paroxysmal Nocturnal Hemoglobinuria
APPLY-PNH: Anti-C5 treatment Experienced Patients With PNH
The efficacy of FABHALTA administered orally in adults with PNH was evaluated in a multi-center, open-label, 24-week, active comparator-controlled trial (APPLY-PNH; NCT04558918).
The study enrolled adults with PNH and residual anemia (hemoglobin <10 g/dL) despite previous treatment with a stable regimen of anti-C5 treatment (either eculizumab or ravulizumab) for at least 6 months prior to randomization.
Ninety-seven patients were randomized in an 8:5 ratio to switch to FABHALTA 200 mg orally twice daily (n=62) or to continue anti-C5 treatment (US-approved and non-US-approved eculizumab product n=23 or US-approved and non-US-approved ravulizumab product n=12) throughout the duration of the 24-week randomized controlled period. Randomization was stratified based on prior anti-C5 treatment and transfusion history within the last 6 months. Following completion of the 24-week randomized controlled period, all patients were eligible to enroll in a 24-week treatment extension period and receive FABHALTA monotherapy. Subsequently, patients were eligible to enter a separate long-term extension study.
Patients were required to be vaccinated against Neisseria meningitidis and recommended to be vaccinated against Streptococcus pneumoniae and Haemophilus influenzae type B. If the patient had not been previously vaccinated or if a booster was required, vaccination was administered at least 2 weeks prior to the first dose of study medication. If FABHALTA treatment was initiated earlier than 2 weeks after vaccination, antibacterial drug prophylaxis was administered.
Demographics and baseline disease characteristics were generally well balanced between treatment groups (see Table 3). The mean time on prior anti-C5 treatment was 3.8 and 4.2 years for the FABHALTA and anti-C5 groups, respectively. The baseline mean PNH RBC clone size (Type II + III) was 64.6% for FABHALTA and 57.4% for the anti-C5 group.
Table 3. Patient Baseline Demographics and Characteristics in APPLY-PNH:
| Parameters | Statistics | FABHALTA (n=62) | Anti-C5 (Eculizumab or Ravulizumab) (n=35) |
| Age (years) | Mean (SD) min, max | 51.7 (16.9) 22, 84 | 49.8 (16.7) 20, 82 |
| Sex Female | n (%) | 43 (69.4) | 24 (68.6) |
| Race White Asian Black or African American | n (%) n (%) n (%) | 48 (77.4) 12 (19.4) 2 (3.2) | 26 (74.3) 7 (20.0) 2 (5.7) |
| Ethnicity Not Hispanic or Latino Hispanic or Latino Not reported/unknown | n (%) n (%) n (%) | 51 (82.3) 8 (12.9) 3 (4.8) | 27 (77.1) 2 (5.7) 6 (17.1) |
| Hemoglobin level (g/dL) | Mean (SD) | 8.9 (0.7) | 8.9 (0.9) |
| LDH level (U/L) | Mean (SD) | 269 (70) | 273 (85) |
| Absolute reticulocyte count (ARC) (10 9/L) | Mean (SD) | 193 (84) | 191 (81) |
| At least one transfusion in 6 months prior to randomization | n (%) | 35 (56.5) | 21 (60.0) |
| History of MAVEs in the last 12 months | n (%) | 12 (19.4) | 10 (28.6) |
| Disease duration (years) | Mean (SD) | 11.9 (9.8) | 13.5 (10.9) |
Abbreviations: LDH, lactate dehydrogenase; MAVEs, major adverse vascular events (includes thrombosis, stroke and myocardial infarction); SD, standard deviation.
Efficacy was established based on demonstration of superiority of switching to FABHALTA compared to continuing on anti-C5 therapy in achieving hematological response after 24 weeks of treatment, without a need for transfusion, by assessing the proportion of patients demonstrating: 1) sustained increase of ≥2 g/dL in hemoglobin levels from baseline (hemoglobin improvement) and 2) sustained hemoglobin levels ≥12 g/dL. Additional efficacy endpoints included transfusion avoidance, change from baseline in hemoglobin levels and change from baseline in absolute reticulocyte counts.
The efficacy results from the APPLY-PNH trial are provided in Table 4.
Table 4. Efficacy Results for the 24-week Randomized Treatment Period for APPLY-PNH:
| Endpoints | FABHALTA (N=62) | Anti-C5 (Eculizumab or Ravulizumab) (N=35) | Difference (95% CI) p-value |
| Primary endpoints | |||
| Patients with sustained increase of hemoglobin levels ≥2 g/dLa from baseline in the absence of transfusions Response rate (%) (95% CI) | 51/62 82.3 (70.5, 90.8) | 0/35 0 (0, 10.0) | 81.5 b (71.6, 91.4) < 0.0001 |
| Patients with sustained hemoglobin level ≥12 g/dLa in the absence of transfusions Response rate (%) (95% CI) | 42/62 67.7 (54.7, 79.1) | 0/35 0 (0, 10.0) | 66.6 b (54.6, 78.6) < 0.0001 |
| Secondary endpoints | |||
| Patients avoiding transfusionc,d Transfusion avoidance rate (%) (95% CI) | 59/62 95.2 (86.5, 99.0) | 16/35 45.7 (28.8, 63.4) | 49.5 b (32.5, 66.6) < 0.0001 |
| Hemoglobin change from baseline (g/dL) (adjusted meane,f) (95% CI) | 3.6 (3.3, 3.9) | -0.1 (-0.5, 0.3) | 3.7 (3.2, 4.1) < 0.0001 |
| Absolute reticulocyte count change from baseline (10 9/L) (adjusted meane) (95% CI) | -116 (-127, -105) | 0 (-13, 14) | -116 (-132, -100) < 0.0001 |
Abbreviations: RR, rate ratio
a Assessed between Day 126 and 168.
b Adjusted difference in proportion.
c Assessed between Day 14 and 168.
d Transfusion avoidance is defined as absence of administration of packed-red blood cell transfusions between Day 14 and 168.
e Adjusted mean assessed between Day 126 and 168.
f Excludes values within 30 days post-transfusion.
APPOINT-PNH: Complement Inhibitor Naïve Patients with PNH
Study APPOINT-PNH (NCT04820530) is a single arm study in adults with PNH who were not previously treated with a complement inhibitor. This study enrolled a total of 40 adults with PNH (RBC clone size ≥10%), hemoglobin <10 g/dL, and LDH >1.5 times upper limit of normal (ULN). All 40 patients received FABHALTA 200 mg orally twice daily during the 24-week open-label core treatment period. Subsequently, patients were eligible to enroll in a 24-week treatment extension period and continue to receive FABHALTA, followed by a separate long-term extension study.
The mean age of the patients was 42.1 years and 42.5% were female. The mean disease duration was 4.7 years. The baseline mean PNH RBC clone size (Type II + III) was 42.7%, mean baseline hemoglobin was 8.2 g/dL, and approximately 70% of patients required a transfusion in the 6 months prior to treatment. The baseline mean LDH level was 1,699 U/L and the mean absolute reticulocyte count was 154 X 10 9/L. About 13% of patients had a history of MAVEs. No patients discontinued from the core treatment period of the study.
In total, 77.5% (95% CI: 61.5%, 89.2%) of patients (31/40) achieved a sustained increase (between Day 126 and Day 168) in hemoglobin levels from baseline of ≥2 g/dL in the absence of RBC transfusions, based on central laboratory hemoglobin values. In a sensitivity analysis, 87.5% (95% CI: 73.2%, 95.8%) of patients (35/40) achieved a sustained increase (between Day 126 and Day 168) in hemoglobin levels from baseline of ≥2 g/dL in the absence of RBC transfusions, including local laboratory hemoglobin values when central laboratory hemoglobin values were not available.
14.2 Immunoglobulin A Nephropathy (IgAN)
The effect of FABHALTA was evaluated in a multicenter, randomized, placebo-controlled, double-blind study (APPLAUSE-IgAN, NCT04578834) in adults with biopsy-proven IgAN, eGFR ≥20 mL/min/1.73 m², and urine protein-to-creatinine ratio (UPCR) ≥1 g/g on a stable dose of maximally-tolerated renin-angiotensin system (RAS) inhibitor therapy with
or without a stable dose of an SGLT2 inhibitor. Patients with other glomerulopathies or those who had been recently treated with systemic immunosuppressants were excluded. Patients were included in either the Main Study Population (eGFR ≥30 mL/min/1.73 m²) or the Severe Renal Impairment population (eGFR ≥20 and <30 mL/min/1.73 m²). Within each group, patients were randomized (1:1) to either FABHALTA 200 mg or placebo twice daily. Rescue immunosuppressive treatment could be initiated per investigator discretion during the trial.
Patients were required to be vaccinated against Neisseria meningitidis and Streptococcus pneumoniae and were recommended to be vaccinated against Haemophilus influenzae type b. If the patient had not been previously vaccinated or if a booster was required, vaccination was administered at least 2 weeks prior to first dosing. If FABHALTA treatment was initiated earlier than 2 weeks after vaccination, antibacterial drug prophylaxis was administered.
The efficacy analysis was based on the first 250 patients with an eGFR ≥30
mL/min/1.73 m² (Main Study Population), who had completed or discontinued the study prior to the Month 9 visit. At baseline, the mean age of these patients was 39 years (range 18 to 74 years); 52% were male, 44% White, 54% Asian, and <1% Black or African American; the mean eGFR was 64 mL/min/1.73 m²; the geometric mean UPCR (sampled from a 24-hr urine collection) was 2.0 g/g, and 12% had a UPCR ≥3.5 g/g. At baseline, 99% of patients were treated with an ACEi or ARB and 13% were on an SGLT2i. Approximately 59% had a history of hypertension, 6% had a history of type 2 diabetes, and 75% had hematuria based on urine dipstick.
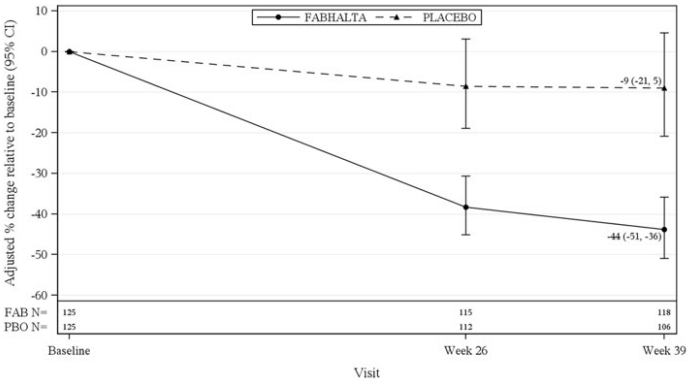
The primary endpoint was the percent reduction in UPCR (sampled from a 24-hr urine collection) at Month 9 relative to baseline (see Table 5). The mean percent change from baseline in UPCR over time is shown in Figure 1.
Table 5. Percent Reduction in UPCR at Month 9 in APPLAUSE-IgAN:
| FABHALTA (N=125) | Placebo (N=125) | |
|---|---|---|
| Geometric mean of UPCR, g/g Baseline Month 9 | 1.9 (n = 125) 1.0 (n = 119) | 2.0 (n = 125) 1.7 (n = 110) |
| % reduction in UPCR at Month 9 relative to baseline (95% CI) | 44% (36%, 51%) | 9% (-5%, 21%) |
| FABHALTA versus placebo: reduction in UPCR at Month 9 relative to baseline (95 CI) | 38% (26%, 49%) | |
| p-valueb | <0.0001 | |
a Percent reduction in UPCR was obtained from the adjusted geometric mean ratios where the log transformed ratio to baseline in UPCR (sampled from 24hr urine collection) was analyzed using an MMRM; Values after taking rescue immunosuppressive treatment for IgAN were imputed to reflect disease worsening. Rescue immunosuppressive treatment for IgAN was initiated in 0 and 7 (5.6%) patients in the FABHALTA and placebo group up to Month 9, respectively.
b One-sided p-value statistically significant at the 0.005 level.
Abbreviations: CI, confidence interval; IgAN, immunoglobulin A nephropathy; MMRM, mixed model of repeated measures; N, number of subjects in each group; n, number of subjects with available data at the time of analysis; UPCR, urine protein-tocreatinine ratio
Figure 1. Geometric Mean Percent Change from Baseline in UPCR by Visit in APPLAUSE-IgAN:
Adjusted % change relative to baseline in UPCR were obtained by analyzing the log transformed ratio to baseline in UPCR using an MMRM as described in Table 5. N represents the number of subjects with values non-missing/not imputed as per the intercurrent event handling strategy by visit and treatment group.
Abbreviations: CI, confidence interval; FAB, FABHALTA; MMRM, mixed model repeated measures; N, number of subjects in each group; PBO, placebo; UPCR, urine protein-tocreatinine ratio.
The treatment effect on UPCR at Month 9 was consistent across all subgroups including age, sex, race, baseline disease characteristics (such as baseline eGFR and proteinuria levels), and the use of SGLT2i.
14.3 Complement 3 Glomerulopathy (C3G)
The efficacy of FABHALTA in reducing proteinuria in adult patients with native kidney C3G was demonstrated in the APPEAR-C3G trial. Safety and effectiveness of FABHALTA in patients with recurrent C3G following kidney transplant have not been established. APPEAR-C3G was a randomized, double-blind, placebo-controlled study in 74 adult patients with biopsy confirmed native kidney C3G who had a urine protein-to-creatinine ratio (UPCR) ≥1 g/g and eGFR ≥30 mL/min/1.73 m² (NCT04817618). Patients were randomized (1:1) to receive either FABHALTA 200 mg orally twice daily (N=38) or placebo (N=36) for 6 months, followed by a 6-month open-label treatment period in which all patients received FABHALTA 200 mg orally twice daily.
Patients were required to be on a maximally tolerated renin-angiotensin system (RAS) inhibitor and could be on a corticosteroid and/or mycophenolate mofetil/sodium (MMF/MPS) at baseline. All background therapies (i.e., RAS inhibitors, corticosteroids and MMF/MPS) were required to be at stable doses for 90 days prior to randomization and throughout the study. Randomization was stratified according to whether patients were receiving concomitant immunosuppressive therapy.
Patients were required to be vaccinated against Neisseria meningitidis and Streptococcus pneumoniae and were recommended to be vaccinated against Haemophilus influenzae type b. If the patient had not been previously vaccinated or if a booster was required, vaccination was administered at least 2 weeks prior to first dosing. If FABHALTA treatment was initiated earlier than 2 weeks after vaccination, antibacterial drug prophylaxis was administered.
At baseline, the mean age of patients was 28 years (range 18 to 60 years), 64% were male, 69% were White, 24% were Asian, and 9% were Hispanic or Latino. The mean baseline eGFR (mL/min/1.73 m²) was 89 and 99 in the FABHALTA and placebo groups, respectively, and the geometric mean 24-hour UPCR (g/g) at baseline was 3.3 and 2.6 in the FABHALTA and placebo groups, respectively.
Twenty four percent of patients in the FABHALTA group and 3% in the placebo group had dense deposit disease. Baseline use of corticosteroids and/or MMF/MPS, and RAS inhibitor was balanced among the FABHALTA and placebo groups. Overall, 45% of patients were on corticosteroids and/or MMF/MPS, and 99% of patients were on a RAS inhibitor at baseline.
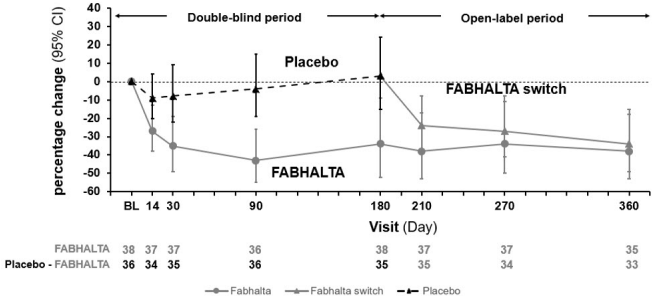
The primary efficacy endpoint was the log-transformed ratio to baseline in UPCR (sampled from a 24-hour urine collection) at 6 months. At 6 months, the geometric mean UPCR ratio relative to baseline was 0.70 (95% CI: 0.57, 0.85) and 1.08 (95% CI: 0.88, 1.31) in the FABHALTA and placebo groups, respectively, resulting in a 35% reduction in 24-hour UPCR from baseline in the FABHALTA group compared to placebo (p=0.0028).
Following the initial 6-month treatment period, all patients were treated with FABHALTA for an additional 6 months. In patients initially randomized to FABHALTA, the reduction in 24-hour UPCR seen at 6 months was maintained at Month 12. In patients who switched from placebo to FABHALTA, the magnitude of the reduction in 24-hour UPCR from Month 6 to 12 was similar to the reduction seen in patients initially randomized to FABHALTA. The geometric mean percent change from baseline in UPCR (measured as first morning void [FMV]) over time is shown in Figure 2.
Compared to patients treated with placebo, patients treated with FABHALTA had a 7-fold higher odds (p=0.0166) of achieving a composite renal endpoint defined as a ≥50% reduction in 24-hour UPCR compared to baseline and stable or improved eGFR compared to baseline [≤15% reduction in eGFR] at 6 months. Although a greater proportion of patients in the FABHALTA arm (30%) as compared to placebo (6%) achieved a ≥50% reduction in 24-hour UPCR compared to baseline, there was no difference between arms in the proportion of patients with stable or improved eGFR compared to baseline at 6 months (90% in FABHALTA vs 89% in placebo).
Figure 2. Geometric Mean Percent Change from Baseline in UPCR FMV (g/g) up to Month 12 (APPEAR-C3G):
The treatment effect of FABHALTA on UPCR at Month 6 was generally consistent across subgroups including age, sex, race, baseline disease characteristics (such as baseline proteinuria and eGFR levels) and use of immunosuppressive therapies.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.