FACTIVE Tablet Ref.[50460] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2016
Product description
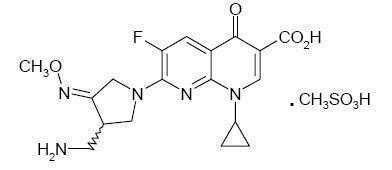
FACTIVE (gemifloxacin mesylate) is a synthetic broad-spectrum antibacterial agent for oral administration. Gemifloxacin, a compound related to the fluoroquinolone class of antibiotics, is available as the mesylate salt in the sesquihydrate form. Chemically, gemifloxacin is (R,S)7[(4Z)3(aminomethyl)4(methoxyimino)-1-pyrrolidinyl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid.
The mesylate salt is a white to light brown solid with a molecular weight of 485.49. Gemifloxacin is considered freely soluble at neutral pH (350 μg/mL at 37ºC, pH 7.0). Its empirical formula is C18H20FN5O4•CH4O3S and its chemical structure is:
Each white to off-white, oval, film-coated FACTIVE tablet has breaklines and GE 320 debossed on both faces and contains gemifloxacin mesylate equivalent to 320 mg gemifloxacin. The inactive ingredients are crospovidone, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, and titanium dioxide.
| How Supplied |
|---|
|
FACTIVE (gemifloxacin mesylate) is available as white to off-white, oval, film-coated tablets with breaklines and GE 320 debossed on both faces. Each tablet contains gemifloxacin mesylate equivalent to 320 mg of gemifloxacin. 320 mg Unit of Use (CR*) 5’s NDC 44001-321-05 * Child Resistant Manufactured for: MERUS LABS, Toronto, ON M5K 1H1 Canada Licensed from LG Life Sciences, Ltd. Seoul, Korea |
Drugs
| Drug | Countries | |
|---|---|---|
| FACTIVE | Brazil, Ecuador, Mexico, Tunisia, Turkey, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.