FARYDAK Hard capsule Ref.[9119] Active ingredients: Panobinostat
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Secura Bio Limited, 32 Molesworth Street, Dublin 2, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Other antineoplastic agents
ATC code: L01XX42
Mechanism of action
Farydak is a histone deacetylase (HDAC) inhibitor that inhibits the enzymatic activity of HDACs at nanomolar concentrations. HDACs catalyse the removal of acetyl groups from the lysine residues of histones and some non-histone proteins. Inhibition of HDAC activity results in increased acetylation of histone proteins, an epigenetic alteration that results in a relaxing of chromatin, leading to transcriptional activation. In vitro, panobinostat caused the accumulation of acetylated histones and other proteins, inducing cell cycle arrest and/or apoptosis of some transformed cells. Increased levels of acetylated histones were observed in xenografts from mice that were treated with panobinostat. Panobinostat shows more cytotoxicity towards tumour cells compared to normal cells.
Pharmacodynamic effects
Treatment of tumour cells with panobinostat resulted in a dose-dependent increase in acetylation of histones H3 and H4 both in vitro and in xenograft animal pre-clinical models, demonstrating target inhibition. In addition, increased expression of the tumour suppressor gene p21CDKNIA (cyclin dependent kinase inhibitor 1/p21) gene, a key mediator of G1 arrest and differentiation, was triggered with panobinostat exposure.
Clinical efficacy and safety
Clinical efficacy in patients with relapsed and relapsed and refractory multiple myeloma (Study D2308 – Panorama 1)
The efficacy and safety of panobinostat in combination with bortezomib and dexamethasone were evaluated in a randomised, double-blind, placebo-controlled, multicentre phase III study in patients with relapsed or relapsed and refractory multiple myeloma who had received 1-3 prior lines of therapies.
Patients received panobinostat (20 mg taken orally once a day, three times per week, on a 2 weeks on and 1 week off dosing regimen), in combination with bortezomib (1.3 mg/m² injected intravenously) and dexamethasone (20 mg). Treatment was administered for a maximum of 16 cycles (see Tables 1 and 2).
A total of 768 patients were randomised in a 1:1 ratio to either the panobinostat + bortezomib + dexamethasone (n=387) or the placebo + bortezomib + dexamethasone (n=381) arm, stratified by prior use of bortezomib [Yes (n=336 (43.8%)), No (n=432 (56.3%))] and number of prior lines of anti-myeloma therapy [1 prior line (n=352 (45.8%)), 2 to 3 prior lines (n=416 (54.2%))]. Demographics and baseline disease characteristics were balanced and comparable between the study arms.
The median age was 63 years, range 28-84; 42.1% of patients were older than 65 years. A total of 53.0% of patients were male. Caucasians comprised 65.0% of the study population, Asians 30.2% and blacks 2.9%. ECOG performance status was 0-1 in 93% of patients. The median number of prior therapies was 1.0. More than half (57.2%) of the patients had undergone prior stem cell transplantation and 62.8% of the patients were relapsed after previous anti-neoplastic therapies (e.g. melphalan 79.6%, dexamethasone 81.1%, thalidomide 51.2%, cyclophosphamide 45.3%, bortezomib 43.0%, combined bortezomib and dexamethasone 37.8%, lenalidomide 20.4%). More than one third (35.8%) of the patients were relapsed and refractory to prior treatment.
The median duration of follow-up was 28.75 months in the panobinostat + bortezomib + dexamethasone arm and 29.04 months in the placebo + bortezomib + dexamethasone arm.
The primary endpoint was progression free survival (PFS) as per modified European Bone Marrow Transplant Group (mEBMT) criteria and as assessed by the investigator. In the overall patient population PFS based on the full analysis set (FAS) was statistically significantly different between the treatment arms (stratified Log-rank test p<0.0001, with an estimated 37% risk reduction in the panobinostat + bortezomib + dexamethasone arm compared to the placebo + bortezomib + dexamethasone arm (Hazard ratio: 0.63 (95% CI: 0.52, 0.76)). The median PFS (95% CI) was 12.0 months (10.3, 12.9) and 8.1 months (7.6, 9.2), respectively.
Overall survival (OS) was the key secondary endpoint. OS was not statistically significantly different between the two treatment groups. The median OS was 40.3 months in the panobinostat + bortezomib + dexamethasone arm and 35.8 months in the placebo + bortezomib + dexamethasone arm (Hazard ratio: 0.94 (95% CI: 0.78, 1.14)).
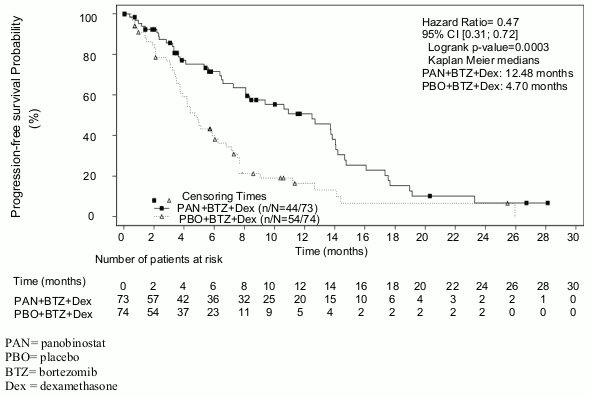
Out of the pre-specified subgroup of patients with prior treatment with bortezomib and an immunomodulatory agent (N=193), 76% of patients had received at least two prior regimens. In this subset of patients (N=147), the median duration of treatment was 4.5 months in the panobinostat + bortezomib + dexamethasone arm and 4.8 months in the placebo + bortezomib + dexamethasone arm. The median PFS (95% CI) was 12.5 months (7.26, 14.03) in the panobinostat + bortezomib + dexamethasone arm and 4.7 months (3.71, 6.05) in the placebo + bortezomib + and dexamethasone arm [HR: 0.47 (0.31, 0.72)]. These patients had a median of 3 prior therapies. Efficacy results are summarised in Table 8 and the Kaplan-Meier curves for PFS are provided in Figure 2.
Table 8. Progression-free survival in patients who received at least two prior regimens including bortezomib and an immunomodulating agent:
| Farydak bortezomib and dexamethasone N=73 | Placebo bortezomib and dexamethasone N=74 | |
|---|---|---|
| Progression-free survival | ||
| Median, months [95% CI] | 12.5 [7.26, 14.03] | 4.7 [3.71, 6.05] |
| Hazard ratio [95% CI]1 | 0.47 (0.31, 0.72) | |
1 Hazard ratio obtained from stratified Cox model
Figure 2. Kaplan-Meier plot of progression-free survival in patients with multiple myeloma who received at least two prior regimens including bortezomib and an immunomodulatory agent:
In the subgroup of patients who had received at least two prior regimens including bortezomib and an immunomodulatory agent (n=147), the overall response rate using modified EBMT criteria was 59% in the panobinostat + bortezomib + dexamethasone arm and 39% in the placebo + bortezomib + dexamethasone arm. Response rates are summarised in Table 9.
Table 9. Response rates in patients with multiple myeloma who received at least two prior regimens including bortezomib and an immunomodulatory agent:
| Farydak bortezomib and dexamethasone N=73 | Placebo bortezomib and dexamethasone N=74 | |
|---|---|---|
| Overall response | 43 (59%) | 29 (39%) |
| [95% CI] | (46.8, 70.3) | (28, 51.2) |
| Complete response | 6 (8%) | 0 |
| Near complete response | 10 (14%) | 6 (8%) |
| Partial response | 27 (37%) | 23 (31%) |
Clinical efficacy in patients with bortezomib-refractory multiple myeloma (Study DUS71 – Panorama 2)
Study DUS71 was a two-stage, single-arm, open-label multicentre phase II study of oral panobinostat (20 mg) in combination with bortezomib (1.3 mg/m²) and dexamethasone (20 mg) in 55 patients with relapsed and refractory multiple myeloma, who were bortezomib-refractory and had received at least two prior lines of therapy. Patients had to be exposed to an IMiD (lenalidomide or thalidomide). Refractoriness to bortezomib was defined as disease progression on or within 60 days of the last bortezomib-containing line of therapy.
The primary endpoint of the study was to assess overall response rate (ORR) after 8 cycles of therapy as per mEBMT criteria.
Patients were heavily pre-treated and had received multiple prior regimens (median: 4; range: 2-11). All 55 patients were previously treated with bortezomib and at least one IMiD (lenalidomide: 98.2%, thalidomide: 69.1%). The majority of patients had received prior transplant (63.6%).
The median duration of exposure to study treatment was 4.6 months (range: 0.1-24.1 months). Patients achieved an ORR (≥PR (partial response)) of 34.5% and 52.7% (≥MR (minimal response)). The median time to response was 1.4 months and the median duration of response was 6.0 months. The median OS was 17.5 months.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Farydak in all subsets of the paediatric population in multiple myeloma (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Panobinostat is rapidly and almost completely absorbed with T max reached within 2 hours of oral administration in patients with advanced cancer. The absolute oral bioavailability of panobinostat was approximately 21%. After oral administration, panobinostat pharmacokinetics appear to be linear in the dose range 10-30 mg, but AUC increases less than proportionally with dose at higher doses.
Overall panobinostat exposure and inter-patient variability remained unchanged with or without food, whereas Cmax was reduced by <45% and Tmax prolonged by 1 to 2.5 hours with food (i.e. both normal and high-fat breakfasts). Since food did not alter overall bioavailability (AUC), panobinostat can be administered regardless of food in cancer patients.
Distribution
Panobinostat is moderately (approximately 90%) bound to human plasma proteins. Its fraction in the erythrocyte is 0.60 in vitro, independent of the concentration. The volume of distribution of panobinostat at steady state (Vss) is approximately 1,000 litres based on final parameter estimates in the population pharmacokinetic analysis.
Biotransformation
Panobinostat is extensively metabolised, and a large fraction of the dose is metabolised before reaching the systemic circulation. Pertinent metabolic pathways involved in the biotransformation of panobinostat are reduction, hydrolysis, oxidation and glucuronidation processes. Oxidative metabolism of panobinostat played a less prominent role, with approximately 40% of the dose eliminated by this pathway. Cytochrome P450 3A4 (CYP3A4) is the main oxidation enzyme, with potential minor involvement of CYP2D6 and 2C19.
Panobinostat represented 6 to 9% of the drug-related exposure in plasma. The parent substance is deemed to be responsible for the overall pharmacological activity of panobinostat.
Elimination
After a single oral dose of [14C] panobinostat in patients, 29 to 51% of administered radioactivity is excreted in the urine and 44 to 77% in the faeces. Unchanged panobinostat accounted for <2.5% of the dose in urine and <3.5% of the dose in faeces. The remainders are metabolites. Apparent panobinostat renal clearance (CLR/F) was found to range from 2.4 to 5.5 l/h. Panobinostat has a terminal elimination half-life of approximately 37 hours based on final parameters estimate in the population PK analysis.
Special populations
Paediatric population
Panobinostat was not evaluated in multiple myeloma patients under 18 years of age.
Elderly population
In the phase III clinical study 162 out of 387 patients were aged 65 years or over. Plasma exposure of panobinostat in patients aged 65 years or younger was similar to those older than 65 years in the pooling of single-agent panobinostat studies between the dose range of 10 mg and 80 mg.
Patients with hepatic impairment
The effect of hepatic impairment on the pharmacokinetics of panobinostat was evaluated in a phase I study, in 24 patients with solid tumours and with varying degrees of hepatic impairment. Mild and moderate hepatic impairment as per NCI-CTEP classification increased panobinostat plasma exposure by 43% and 105%, respectively. No pharmacokinetic data are available for patients with severe hepatic impairment.
Patients with renal impairment
The effect of renal impairment on the pharmacokinetics of panobinostat was assessed in a phase I study in 37 patients with advanced solid tumours with varying degrees of renal function. Mild, moderate and severe renal impairment based on baseline urinary creatinine clearance did not increase the panobinostat plasma exposure in mild, moderate and severe groups.
Preclinical safety data
Repeated dose toxicity studies
The primary target organs of toxicity following administration of panobinostat in rats and dogs were identified as the erythropoietic, myelopoietic and lymphatic systems. The thyroid changes including hormones in dogs (decrease triodothyronine (T3)) and rats (decrease in triodothyronine (T3), tetraiodothyronine (T4) (males) and thyroid stimulating hormone (TSH)) were observed at exposures corresponding to 0.07-2.2 of the human AUC observed clinically.
Carcinogenesis and mutagenesis
Carcinogenicity studies have not been performed with panobinostat. Panobinostat has demonstrated mutagenic potential in the Ames assay, endo-reduplication effects in human peripheral blood lymphocytes in vitro, and DNA damage in an in vivo COMET study in mouse lymphoma L5178Y cells, that are attributed to the pharmacological mode of action.
Reproduction toxicity
An increase in early resorptions was observed in female rats (doses ≥30 mg/kg). Prostatic atrophy accompanied by reduced secretory granules, testicular degeneration, oligospermia and increased epididymal debris were observed in dogs at exposures corresponding to 0.41-0.69 of the human clinical AUC and not fully reversible after a 4 week recovery period.
Based on animal data, the likelihood of panobinostat increasing the risk of foetal death and developmental skeletal abnormalities is predicted to be high. Embryo foetal lethality and increases in skeletal anomalies (extra sternabrae, extra ribs, increases in minor skeletal variations, delayed ossification and variations of the sternabrae) were seen above exposures corresponding to 0.25 of the human clinical AUC.
The effects of panobinostat on labour and post-natal growth and maturation were not evaluated in animal studies.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.