FETZIMA Extended release capsule Ref.[10811] Active ingredients: Milnacipran
Source: FDA, National Drug Code (US) Revision Year: 2019
12.1. Mechanism of Action
The exact mechanism of the antidepressant action of levomilnacipran is unknown, but is thought to be related to the potentiation of serotonin and norepinephrine in the central nervous system, through inhibition of reuptake at serotonin and norepinephrine transporters. Non-clinical studies have shown that levomilnacipran is a potent and selective serotonin and norepinephrine reuptake inhibitor (SNRI).
12.2. Pharmacodynamics
Levomilnacipran binds with high affinity to the human serotonin (5-HT) and norepinephrine (NE) transporters (Ki = 11 and 91 nM, respectively) and potently inhibits 5-HT and NE reuptake (IC50 = 16-19 and 11 nM, respectively). Levomilnacipran lacks significant affinity for any other receptors, ion channels or transporters tested in vitro, including serotonergic (5HT1-7), α- and β-adrenergic, muscarinic, or histaminergic receptors and Ca2+, Na+, K+ or Cl-channels. Levomilnacipran did not inhibit monoamine oxidase (MAO).
Cardiovascular Electrophysiology
At a dose 2.5 times the maximum recommended dose, levomilnacipran does not prolong QTc to any clinically relevant extent.
12.3. Pharmacokinetics
The concentration of levomilnacipran at steady state is proportional to dose when administered from 25 to 300 mg once daily. Following an oral administration, the mean apparent total clearance of levomilnacipran is 21-29 L/h. Steady-state concentrations of levomilnacipran are predictable from single-dose data. The apparent terminal elimination half-life of levomilnacipran is approximately 12 hours. After daily dosing of FETZIMA 120 mg, the mean Cmax value is 341 ng/mL, and the mean steady-state AUC value is 5196 ng·h/mL. Interconversion between levomilnacipran and its stereoisomer does not occur in humans.
Absorption
The relative bioavailability of levomilnacipran after administration of FETZIMA was 92% when compared to oral solution. Levomilnacipran concentration was not significantly affected when FETZIMA was administered with food.
The median time to peak concentration (Tmax) of levomilnacipran is 6-8 hours after oral administration.
Distribution
Levomilnacipran is widely distributed with an apparent volume of distribution of 387-473 L; plasma protein binding is 22% over concentration range of 10 to 1000 ng/mL.
Elimination
Metabolism
Levomilnacipran undergoes desethylation to form desethyl levomilnacipran and hydroxylation to form p-hydroxy-levomilnacipran. Both oxidative metabolites undergo further conjugation with glucuronide to form conjugates. The desethylation is catalyzed primarily by CYP3A4 with minor contribution by CYP2C8, 2C19, 2D6, and 2J2.
Excretion
Levomilnacipran and its metabolites are eliminated primarily by renal excretion. Following oral administration of 14C-levomilnacipran solution, approximately 58% of the dose is excreted in urine as unchanged levomilnacipran. N-desethyl levomilnacipran is the major metabolite excreted in the urine and accounted for approximately 18% of the dose. Other identifiable metabolites excreted in the urine are levomilnacipran glucuronide (4%), desethyl levomilnacipran glucuronide (3%), p-hydroxy levomilnacipran glucuronide (1%), and p-hydroxy levomilnacipran (1%). The metabolites are inactive [see Dosage and Administration (2.3)].
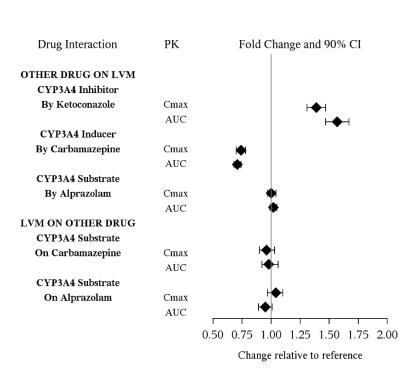
Drug Interaction Studies
The drug interaction studies for levomilnacipran are summarized in Figure 1.
In vitro Studies
In vitro studies suggested that CYP2C8, CYP2C19, CYP2D6, and CYP2J2 had minimal contributions to metabolism of levomilnacipran. In addition, levomilnacipran is not a substrate of BCRP, OATP1B1, OATP1B3, OAT1, OAT3, or OCT2 and is a weak substrate of P-gp.
In vitro studies have shown that levomilnacipran is not an inhibitor of CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, P-gp, OATP1B1, OATP1B3, OAT1, OAT3, or OCT2.
Alcohol
An in vitro study indicated increases of levomilnacipran release from Fetzima extended-release capsules (20, 40, 80, and 120 mg) at 2 hours by approximately 9.5%, 23% and 56% in the presence of 5%, 20% and 40% (v/v) alcohol, respectively. Effect of 40% alcohol resulted in nearly complete drug release in 4 hours. There is no in vivo study conducted for the effect of alcohol on drug exposure.
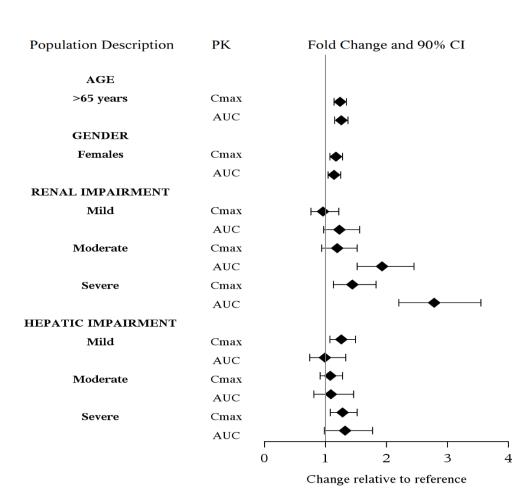
Specia Populations
The effect of intrinsic patient factors on the pharmacokinetics of levomilnacipran is presented in Figure 2.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Levomilnacipran administered by oral gavage to rats for 2 years and Tg.rasH2 mice for 6 months did not increase the incidence of tumors in either study.
Rats received levomilnacipran at doses up to 90/70 mg/kg/day (the dose was lowered in males after 45 weeks of dosing). The 90 mg/kg/day dose is 7 times the maximum recommended human dose (MRHD) of 120 mg on a mg/m² basis.
Tg.rasH2 mice received levomilnacipran at doses up to 150 mg/kg/day.
Mutagenesis
Levomilnacipran was not mutagenic in the in vitro bacterial mutation assay (Ames test) and was not clastogenic in an in vivo micronucleus assay in rats. Additionally, levomilnacipran was not genotoxic in the in vitro mouse lymphoma (L5178Y TK+/-) cell forward mutation assay.
Impairment of Fertility
When levomilnacipran was administered orally to male and female rats before mating, through mating and up to Day 7 of gestation at doses up to 100 mg/kg/day, no effects were observed on fertility. This dose is 8 times the MRHD on a mg/m² basis.
14. Clinical Studies
14.1 Treatment of Major Depressive Disorder
The efficacy of FETZIMA for the treatment of major depressive disorder (MDD) was established in three 8-week randomized, double-blind, placebo-controlled studies (at doses 40-120 mg once daily) in adult (18-78 years of age) outpatients who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for MDD. Two of the studies were fixed dose (Study 1 and Study 2) and one study was flexible dose (Study 3).
In Study 1, patients received 40 mg (n=178), 80 mg (n=179), or 120 mg (n=180) of FETZIMA once daily, or placebo (n=176). In Study 2, patients received either 40 mg (n=188) or 80 mg (n=188) of FETZIMA once daily, or placebo (n=186). In the flexible-dose study (Study 3), patients received 40 to 120 mg (n=217) of FETZIMA once daily, or placebo (n=217) with 21%, 34%, and 44% of FETZIMA patients on 40 mg, 80 mg, and 120 mg, respectively at the end of their treatment.
In all three studies, FETZIMA demonstrated superiority over placebo in the improvement of depressive symptoms as measured by the Montgomery-Asberg Depression Rating Scale (MADRS) total score (see Table 6). FETZIMA also demonstrated superiority over placebo as measured by improvement in the Sheehan Disability Scale (SDS) functional impairment total score.
Table 6. Summary of Results for the Primary Efficacy Endpoint MADRS:
| Study Number | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Differencea (95% CI) |
|---|---|---|---|---|
| Study 1 (fixed dose) | FETZIMA (ER 40 mg/day)* | 36.0 (4.1) | -14.8 (1.0) | -3.2 (-5.9, -0.5) |
| FETZIMA (ER 80 mg/day)* | 36.1 (3.9) | -15.6 (1.0) | -4.0 (-6.7, -1.3) | |
| FETZIMA (ER 120 mg/day)* | 36.0 (3.9) | -16.5 (1.0) | -4.9 (-7.6, -2.1) | |
| Placebo | 35.6 (4.5) | -11.6 (1.0) | -- | |
| Study 2 (fixed-dose) | FETZIMA (ER 40 mg/day)* | 30.8 (3.4) | -14.6 (0.8) | -3.3 (-5.5, -1.1) |
| FETZIMA (ER 80 mg/day)* | 31.2 (3.5) | -14.4 (0.8) | -3.1 (-5.3, -1.0) | |
| Placebo | 31.0 (3.8) | -11.3 (0.8) | -- | |
| Study 3 (flexible-dose) | FETZIMA (ER 40 - 120 mg/day)* | 35.0 (3.6) | -15.3 (0.8) | -3.1 (-5.3, -0.9) |
| Placebo | 35.2 (3.8) | -12.2 (0.8) | -- |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval unadjusted for multiplicity.
a Difference (drug minus placebo) in least-squares mean change from baseline to endpoint (Week 8).
* Doses statistically significantly superior to placebo.
Post-hoc analyses of the relationships between treatment outcome and age, gender, and race did not suggest any differential responsiveness on the basis of these patient characteristics.
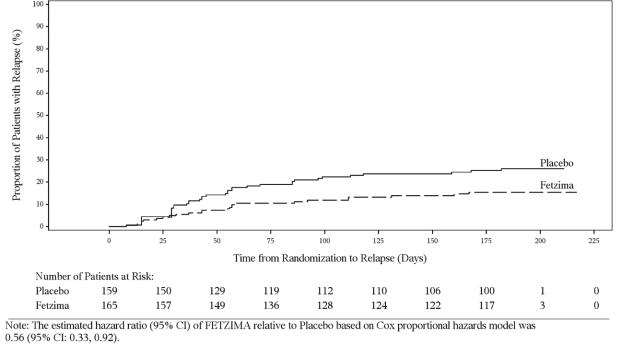
In a maintenance study (Study 4; NCT02288325), adult patients meeting DSM-5 criteria for MDD received flexibly dosed FETZIMA (40 mg, 80 mg, or 120 mg) once daily for 8 weeks. Responders in the initial 8-week treatment period were eligible to enter a 12-week, open-label, fixed-dose stabilization phase. At the end of stabilization, approximately half of the subjects were receiving 120 mg once daily. Three hundred twenty-four (324) patients who met the response criteria (MADRS total score ≤12) after 20 weeks of open-label treatment were randomized to the double-blind treatment phase with FETZIMA or placebo for 26 weeks. The primary efficacy endpoint was the time from randomization to first relapse during the double-blind phase. Relapse of depressive episode was defined as a MADRS total score ≥18 at two consecutive visits or insufficient therapeutic response as judged by the investigator. Patients on FETZIMA experienced a statistically significantly longer time to relapse than patients on placebo (Figure 3).
Figure 3. Kaplan-Meier Estimated Proportion of Patients with Relaps e for Adults with MDD (Study 4):
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.