FIBROVEIN Solution for injection Ref.[7675] Active ingredients: Sodium tetradecyl sulfate
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: STD Pharmaceutical Products Ltd, Plough Lane, Hereford, HR4 0EL, United Kingdom
Therapeutic indications
For the treatment of uncomplicated primary varicose veins, recurrent or residual varicose veins following surgery, reticular veins, venules and spider veins of the lower extremities that show simple dilation.
Posology and method of administration
Posology
Fibrovein is for intravenous use only. The strength of solution required depends on the size and degree of varicosity. Spider veins should only be treated with the 0.2%, reticular veins with 0.5%, the 1% solution will be found most useful for small to medium varicosities and the 3% solution for larger varicosities. The size of non-visible varicose veins should be measured under ultrasound.
The sclerosant should be administered intravenously in small aliquots at multiple sites along the vein to be treated either as a liquid or as a sclerosant/air mixture (foam), for the treatment of larger veins with the 1% and 3% solutions. The objective is to achieve optimal destruction of the vessel wall with the minimum concentration of sclerosant necessary for a clinical result. If the concentration is too high necrosis or other adverse sequelae may occur.
Adults
| Concentration | Normal volume injected intravenously at suitable sites per session | Maximum total volume to be injected per session | ||
|---|---|---|---|---|
| Liquid | Foam* | Liquid | Foam* | |
| Fibrovein 3% | 0.5 to 2.0 ml | 0.5 to 2.0 ml | 4ml | 16 ml |
| Fibrovein 1% | 0.1 to 1.0 ml | 0.5 to 2.0 ml | 10 ml | 16 ml |
| Fibrovein 0.5% and 0.2% | 0.1 to 1.0 ml | NA | 10 ml | NA |
* volume is the sum of the liquid and air components
Where special caution is indicated it is recommended that a test dose of 0.25 to 0.5 ml Fibrovein should be given followed by observation of the patient for several hours before administration of a second or larger dose.
As the volume to be injected is limited per session, repeated sessions are usually needed (2 to 4 on average). To prevent a possible allergic reaction, it is recommended that a small test dose of Fibrovein should be given at the beginning of each session.
When the sclerosant is administered as a foam
Fibrovein 3% and 1% may be converted to a foam to be used for the treatment of larger veins. The foam must be prepared just before use and administered by a physician appropriately trained in the correct generation and administration of foam. It should ideally be administered under ultra sound guidance.
Elderly population
No specific dose recommendations apply.
Paediatric population
The safety and efficacy of Fibrovein in children and adolescents have not been established. No data are available.
Method of administration
The Tessari method of preparation of the foam is described in section 6.6. Other techniques may be used e.g. DSS, Easyfoam and Steriven most of which consist of mixing sclerosant and sterile air by repeated passes through 2 connected syringes. Specific instructions for handling are detailed in section 6.6.
Strict aseptic technique must be maintained while handling Fibrovein.
Fibrovein is a single-use parenteral product. Once the container is opened, use immediately and discard any unused portion.
Visually inspect for particulate matter before use. Solutions that contain particulate matter should not be used.
Overdose
No case of systemic overdose has been reported. Using a higher concentration than recommended in small veins may lead to pigmentation and/or local tissue necrosis.
Shelf life
Shelf life: 3 years.
After first opening, the medicinal product should be used immediately.
Special precautions for storage
This medicinal product does not require any special temperature conditions (Temperature Zone 1).
Do not store above 25°C (For all other Temperature zones).
Do not freeze.
Keep the vial/ampoule in the outer carton in order to protect from light.
Nature and contents of container
2ml ampoule (Type I glass).
5ml vial (Type 1 glass) with a stopper (chlorobutyl) and aluminium seal with flip-off cap (polypropylene).
Pack size of 5 ampoules of 2 ml or 2, 5 or 10 vials of 5 ml.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Fibrovein 3% and 1% Solution for Injection
The foam must be prepared just before use and administered by a physician appropriately trained in the correct generation and administration of foam.
Strict aseptic technique must be maintained while manufacturing the foam.
General guidelines
The quality of foam depends on specific criteria:
- The product concentration: Foam can only be prepared with concentrations of 1 to 3 % sodium tetradecyl sulfate.
- The proportion of liquid to air: Usually, this proportion is 1 volume of liquid for 3 volumes of air.
- Number of backwards and forwards passes: The physician should follow precisely the number of movements defined for each technique.
- Macroscopic consistency of the foam: The quality of the foam should be checked outside of the syringe before administration. The foam should be homogenous, soft and cohesive with no visible large bubbles. If large bubbles are visible, the foam should be thrown away and a new foam prepared.
- The total time of preparation of the foam: The preparation should take around 10 seconds from the first to the last backwards and forwards movement.
- The maximum time between preparation and injection: The sclerosant foam must be used within sixty seconds of production. After sixty seconds, any remaining foam should be discarded. More foam should be prepared if required.
Manufacture of foam using the Tessari technique
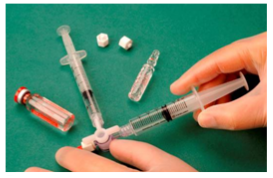
To create the foam 1 ml of liquid sclerosant is drawn into a sterile syringe and 3 ml or 4 ml of sterile air is drawn into another sterile syringe. The air is drawn through a 0.2 μm filter to ensure it is sterile. The syringes are then connected using a sterile three way tap/valve (Fig. 1).
The use of Luer lock syringes and eye protection are recommended when making the foam. The connection with the 3-way tap can fail under pressure with Luer slip syringes resulting in product being squirted out uncontrollably.
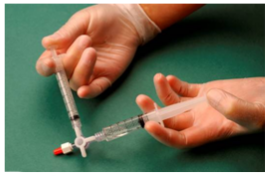
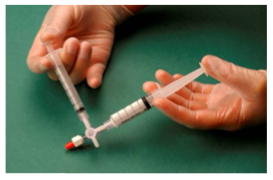
The sclerosant/air mixture is then forced back and forth from one syringe to the other through the 3-way valve at least 20 times to produce a smooth, consistent foam (Fig. 2&3).
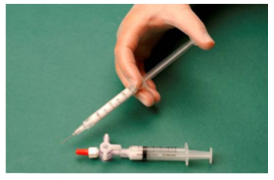
The syringe containing the foam, is then removed and the vein is injected immediately (Fig. 4).
The sclerosant foam must be used within sixty seconds of production. After sixty seconds any remaining foam should be discarded. More foam should be prepared if required.
The quality of the foam should be checked before administration. It should appear homogeneous with no large bubbles visible to the naked eye.
Figure 1:
Figure 2:
Figure 3:
Figure 4:
Disposal
No special requirements for disposal.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.