FIRMAGON Powder and solvent for solution for injection Ref.[8332] Active ingredients: Degarelix
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Ferring Pharmaceuticals A/S, Amager Strandvej 405, 2770 Kastrup, Denmark, Tel: +45 88 33 88 34
Therapeutic indications
FIRMAGON is a gonadotrophin releasing hormone (GnRH) antagonist indicated:
- for treatment of adult male patients with advanced hormone-dependent prostate cancer.
- for treatment of high-risk localised and locally advanced hormone dependent prostate cancer in combination with radiotherapy.
- as neo-adjuvant treatment prior to radiotherapy in patients with high-risk localised or locally advanced hormone dependent prostate cancer.
Posology and method of administration
Posology
| Starting dose | Maintenance dose – monthly administration |
|---|---|
| 240 mg administered as two consecutive subcutaneous injections of 120 mg each | 80 mg administered as one subcutaneous injection |
The first maintenance dose should be given one month after the starting dose.
FIRMAGON may be used as neo-adjuvant or adjuvant therapy in combination with radiotherapy in high-risk localised and locally advanced prostate cancer.
The therapeutic effect of degarelix should be monitored by clinical parameters and prostate specific antigen (PSA) serum levels. Clinical studies have shown that testosterone (T) suppression occurs immediately after administration of the starting dose with 96% of the patients having serum testosterone levels corresponding to medical castration (T≤0.5 ng/ml) after three days and 100% after one month. Long term treatment with the maintenance dose up to 1 year shows that 97% of the patients have sustained suppressed testosterone levels (T≤0.5 ng/ml).
In case the patient's clinical response appears to be sub-optimal, it should be confirmed that serum testosterone levels are remaining sufficiently suppressed. Since degarelix does not induce a testosterone surge it is not necessary to add an anti-androgen as surge protection at initiation of therapy.
Special populations
Elderly, hepatically or renally impaired patients
There is no need to adjust the dose for the elderly or in patients with mild or moderate liver or kidney function impairment (see section 5.2). Patients with severe liver or kidney impairment have not been studied and caution is therefore warranted (see section 4.4).
Paediatric population
There is no relevant use of FIRMAGON in children and adolescents in the treatment of adult male patients with advanced hormone-dependent prostate cancer.
Method of administration
FIRMAGON must be reconstituted prior to administration. For instructions on reconstitution and administration, please see section 6.6.
FIRMAGON is for subcutaneous use ONLY, not to be administered intravenously. Intramuscular administration is not recommended as it has not been studied.
FIRMAGON is administered as a subcutaneous injection in the abdominal region. The injection site should vary periodically. Injections should be given in areas where the patient will not be exposed to pressure e.g. not close to waistband or belt and not close to the ribs.
Overdose
There is no clinical experience with the effects of an acute overdose with degarelix. In the event of an overdose the patient should be monitored and appropriate supportive treatment should be given, if considered necessary.
Shelf life
3 years.
After reconstitution:
Chemical and physical in-use stability has been demonstrated for 2 hours at 25ºC. From a microbiological point of view, unless the method of reconstitution precludes the risk of microbial contamination, the product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user.
Special precautions for storage
This medicinal product does not require any special storage conditions.
For storage conditions of the reconstituted medicinal product, see section 6.3.
Nature and contents of container
FIRMAGON 80 mg powder and solvent for solution for injection:
Glass (type I) vial with bromobutyl rubber stopper and aluminium flip-off seal containing 80 mg powder for solution for injection.
Pre-filled glass (type I) syringe with elastomer plunger stopper, tip cap and line-marking at 4 ml containing 4.2 ml solvent.
Plunger rod.
Vial adapter.
Injection needle (25G 0.5 x 25 mm).
FIRMAGON 120 mg powder and solvent for solution for injection:
Glass (type I) vial with bromobutyl rubber stopper and aluminium flip-off seal containing 120 mg powder for solution for injection.
Pre-filled glass (type I) syringe with elastomer plunger stopper, tip cap and line-marking at 3 ml containing 3 ml solvent.
Plunger rods.
Vial adapters.
Injection needles (25G 0.5 x 25 mm).
Pack sizes
FIRMAGON 80 mg powder and solvent for solution for injection:
Pack-size of 1 tray containing 1 powder vial, 1 solvent pre-filled syringe, 1 plunger rod, 1 vial adapter and 1 needle.
Pack-size of 3 trays containing 3 powder vials, 3 solvent pre-filled syringes, 3 plunger rods, 3 vial adapters and 3 needles.
FIRMAGON 120 mg powder and solvent for solution for injection:
Pack-size of 2 trays containing 2 powder vials, 2 solvent pre-filled syringes, 2 plunger rods, 2 vial adapters and 2 needles.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
The instructions for reconstitution must be followed carefully.
Administration of other concentrations is not recommended because the gel depot formation is influenced by the concentration. The reconstituted solution should be a clear liquid, free of undissolved matter.
NOTE: THE VIALS SHOULD NOT BE SHAKEN
The pack contains one vial of powder and one pre-filled syringe with solvent that must be prepared for subcutaneous injection.
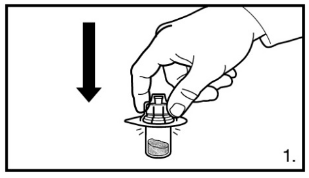
1. Remove the cover from the vial adapter pack. Attach the adapter to the powder vial by pressing the adapter down until the spike pushes through the rubber stopper and the adapter snaps in place.
2. Prepare the pre-filled syringe by attaching the plunger rod.
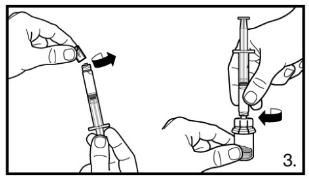
3. Remove the cap of the pre-filled syringe. Attach the syringe to the powder vial by screwing it on to the adapter. Transfer all solvent to the powder vial.
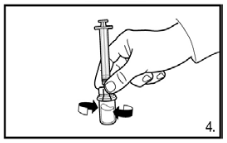
4. With the syringe still attached to the adapter, swirl gently until the liquid looks clear and without undissolved powder or particles. If the powder adheres to the side of the vial above the liquid surface, the vial can be tilted slightly. Avoid shaking to prevent foam formation.
A ring of small air bubbles on the surface of the liquid is acceptable. The reconstitution procedure usually takes a few minutes, but may take up to 15 minutes in some cases.
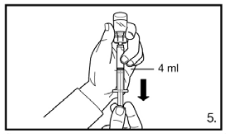
5. Turn the vial upside down and draw up to the line mark on the syringe for injection.
Always make sure to withdraw the precise volume and adjust for any air bubbles.
FIRMAGON 80 mg powder and solvent for solution for injection: withdrawn until the line marking at 4 ml.
FIRMAGON 120 mg powder and solvent for solution for injection: withdrawn until the line marking at 3 ml.
6. Detach the syringe from the vial adapter and attach the needle for deep subcutaneous injection to the syringe.
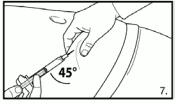
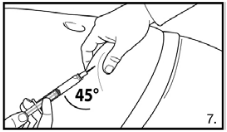
7. Perform a deep subcutaneous injection. To do so: grasp the skin of the abdomen, elevate the subcutaneous tissue and insert the needle deeply at an angle of not less than 45 degrees.
FIRMAGON 80 mg powder and solvent for solution for injection: Inject 4 ml of FIRMAGON 80 mg slowly, immediately after reconstitution.
FIRMAGON 120 mg powder and solvent for solution for injection: Inject 3 ml of FIRMAGON 120 mg slowly, immediately after reconstitution.
8. No injections should be given in areas where the patient will be exposed to pressure, e.g. around the belt or waistband or close to the ribs.
Do not inject directly into a vein. Gently pull back the plunger to check if blood is aspirated. If blood appears in the syringe, the medicinal product can no longer be used. Discontinue the procedure and discard the syringe and the needle (reconstitute a new dose for the patient).
9. FIRMAGON 120 mg powder and solvent for solution for injection
Repeat the reconstitution procedure for the second dose. Choose a different injection site and inject 3 ml.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.