FLAREX Eye drops, suspension Ref.[10812] Active ingredients: Fluorometholone
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
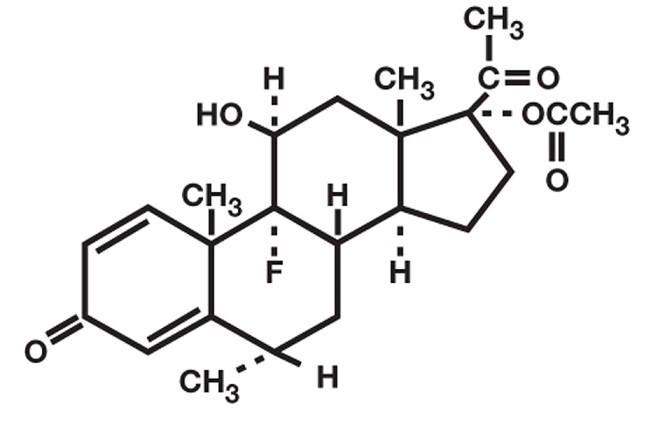
FLAREX (fluorometholone acetate ophthalmic suspension) is a corticosteroid prepared as a sterile topical ophthalmic suspension. The active ingredient, fluorometholone acetate, is a white to creamy white powder with an empirical formula of C24H31FO5 and a molecular weight of 418.5. Its chemical name is 9-fluoro-11β, 17-dihydroxy-6α-methylpregna-1, 4-diene-3, 20-dione 17-acetate.
The chemical structure of Fluorometholone Acetate is presented below:
Each mL contains:
Active: fluorometholone acetate 1 mg (0.1%).
Preservative: benzalkonium chloride 0.01%.
Inactives: sodium chloride, monobasic sodium phosphate, edetate disodium, hydroxyethyl cellulose, tyloxapol, hydrochloric acid and/or sodium hydroxide (to adjust pH), and purified water.
The pH of the suspension is approximately 7.3, with an osmolality of approximately 300 mOsm/kg.
| How Supplied |
|---|
|
FLAREX (fluorometholone acetate ophthalmic suspension) is supplied in white low density polyethylene (LDPE) bottles, with natural LDPE dispensing plugs and pink polypropylene closures. The product is supplied as 5mL in an 8 mL bottle. 5 mL: NDC 71776-100-05 Manufactured for: Eyevance Pharmaceuticals, LLC, Fort Worth, TX 76102 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| FLAREX | Australia, Canada, Hong Kong, Lithuania, Malta, Poland, Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.