FLOVENT DISKUS Inhalation powder Ref.[10814] Active ingredients: Fluticasone
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Fluticasone propionate is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. Fluticasone propionate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor that is 18 times that of dexamethasone, almost twice that of beclomethasone-17-monopropionate (BMP), the active metabolite of beclomethasone dipropionate, and over 3 times that of budesonide. Data from the McKenzie vasoconstrictor assay in man are consistent with these results. The clinical significance of these findings is unknown.
Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of actions on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) involved in inflammation. These anti-inflammatory actions of corticosteroids contribute to their efficacy in asthma.
Though effective for the treatment of asthma, corticosteroids do not affect asthma symptoms immediately. Individual patients will experience a variable time to onset and degree of symptom relief. Maximum benefit may not be achieved for 1 to 2 weeks or longer after starting treatment. When corticosteroids are discontinued, asthma stability may persist for several days or longer.
Trials in subjects with asthma have shown a favorable ratio between topical anti-inflammatory activity and systemic corticosteroid effects with recommended doses of orally inhaled fluticasone propionate. This is explained by a combination of a relatively high local anti-inflammatory effect, negligible oral systemic bioavailability (<1%), and the minimal pharmacological activity of the only metabolite detected in man.
12.2. Pharmacodynamics
In clinical trials with fluticasone propionate inhalation powder using dosages up to and including 250 mcg twice daily, occasional abnormal short cosyntropin tests (peak serum cortisol <18 mcg/dL assessed by radioimmunoassay) were noted both in subjects receiving fluticasone propionate and in subjects receiving placebo. The incidence of abnormal tests at 500 mcg twice daily was greater than placebo. In a 2-year trial carried out with the DISKHALER inhalation device in 64 subjects with mild, persistent asthma (mean FEV1 91% of predicted) randomized to fluticasone propionate 500 mcg twice daily or placebo, no subject receiving fluticasone propionate had an abnormal response to 6-hour cosyntropin infusion (peak serum cortisol <18 mcg/dL). With a peak cortisol threshold of <35 mcg/dL, 1 subject receiving fluticasone propionate (4%) had an abnormal response at 1 year; repeat testing at 18 months and 2 years was normal. Another subject receiving fluticasone propionate (5%) had an abnormal response at 2 years. No subject on placebo had an abnormal response at 1 or 2 years.
In a placebo-controlled clinical trial conducted in subjects aged 4 to 11 years, a 30-minute cosyntropin stimulation test was performed in 41 subjects after 12 weeks of dosing with 50 or 100 mcg twice daily of fluticasone propionate via the DISKUS inhaler. One subject receiving fluticasone propionate via the DISKUS inhaler had a prestimulation plasma cortisol concentration <5 mcg/dL, and 2 subjects had a rise in cortisol of <7 mcg/dL. However, all poststimulation values were >18 mcg/dL.
The potential systemic effects of inhaled fluticasone propionate on the HPA axis were also studied in subjects with asthma. Fluticasone propionate given by inhalation aerosol at dosages of 220, 440, 660, or 880 mcg twice daily was compared with placebo or oral prednisone 10 mg given once daily for 4 weeks. For most subjects, the ability to increase cortisol production in response to stress, as assessed by 6-hour cosyntropin stimulation, remained intact with inhaled fluticasone propionate treatment. No subject had an abnormal response (peak serum cortisol <18 mcg/dL) after dosing with placebo or fluticasone propionate 220 mcg twice daily. For subjects treated with 440, 660, and 880 mcg twice daily, 10%, 16%, and 12%, respectively, had an abnormal response as compared with 29% of subjects treated with prednisone.
12.3. Pharmacokinetics
Absorption
Fluticasone propionate acts locally in the lung; therefore, plasma levels do not predict therapeutic effect. Trials using oral dosing of labeled and unlabeled drug have demonstrated that the oral systemic bioavailability of fluticasone propionate is negligible (<1%), primarily due to incomplete absorption and presystemic metabolism in the gut and liver. In contrast, the majority of the fluticasone propionate delivered to the lung is systemically absorbed. The absolute bioavailability of fluticasone propionate from the DISKUS inhaler in healthy volunteers averages 7.8%.
Peak steady-state fluticasone propionate plasma concentrations in adult subjects with asthma (N=11) ranged from undetectable to 266 pg/mL after a 500-mcg twice-daily dose of fluticasone propionate inhalation powder using the DISKUS inhaler. The mean fluticasone propionate plasma concentration was 110 pg/mL.
Distribution
Following intravenous administration, the initial disposition phase for fluticasone propionate was rapid and consistent with its high lipid solubility and tissue binding. The volume of distribution averaged 4.2 L/kg.
The percentage of fluticasone propionate bound to human plasma proteins averages 99%. Fluticasone propionate is weakly and reversibly bound to erythrocytes and is not significantly bound to human transcortin.
Metabolism
The total clearance of fluticasone propionate is high (average, 1,093 mL/min), with renal clearance accounting for <0.02% of the total. The only circulating metabolite detected in man is the 17β-carboxylic acid derivative of fluticasone propionate, which is formed through the CYP3A4 pathway. This metabolite had less affinity (approximately 1/2,000) than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
Elimination
Following intravenous dosing, fluticasone propionate showed polyexponential kinetics and had a terminal elimination half-life of approximately 7.8 hours. Less than 5% of a radiolabeled oral dose was excreted in the urine as metabolites, with the remainder excreted in the feces as parent drug and metabolites.
Specific Populations
Male and Female Patients
Full pharmacokinetic profiles were obtained from 9 female and 16 male subjects given 500 mcg twice daily. No overall differences in fluticasone propionate pharmacokinetics were observed.
Pediatric Patients
In a clinical trial conducted in subjects aged 4 to 11 years with mild to moderate asthma, fluticasone propionate concentrations were obtained in 61 subjects at 20 and 40 minutes after dosing with 50 and 100 mcg twice daily of fluticasone propionate inhalation powder using the DISKUS. Plasma concentrations were low and ranged from undetectable (about 80% of the plasma samples) to 88 pg/mL. Mean peak fluticasone propionate plasma concentrations at the 50- and 100-mcg dose levels were 5 and 8 pg/mL, respectively.
Patients with Hepatic and Renal Impairment
Formal pharmacokinetic studies using FLOVENT DISKUS have not been conducted in patients with hepatic or renal impairment. However, since fluticasone propionate is predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of fluticasone propionate in plasma. Therefore, patients with hepatic disease should be closely monitored.
Drug Interaction Studies
Inhibitors of Cytochrome P450 3A4: Ritonavir
Fluticasone propionate is a substrate of CYP3A4. Coadministration of fluticasone propionate and the strong CYP3A4 inhibitor ritonavir is not recommended based upon a multiple-dose, crossover drug interaction trial in 18 healthy subjects. Fluticasone propionate aqueous nasal spray (200 mcg once daily) was coadministered for 7 days with ritonavir (100 mg twice daily). Plasma fluticasone propionate concentrations following fluticasone propionate aqueous nasal spray alone were undetectable (<10 pg/mL) in most subjects, and when concentrations were detectable, peak levels (Cmax) averaged 11.9 pg/mL (range: 10.8 to 14.1 pg/mL) and AUC(0-τ) averaged 8.43 pg•h/mL (range: 4.2 to 18.8 pg•h/mL). Fluticasone propionate Cmax and AUC(0-τ) increased to 318 pg/mL (range: 110 to 648 pg/mL) and 3,102.6 pg•h/mL (range: 1,207.1 to 5,662.0 pg•h/mL), respectively, after coadministration of ritonavir with fluticasone propionate aqueous nasal spray. This significant increase in plasma fluticasone propionate exposure resulted in a significant decrease (86%) in serum cortisol AUC.
Ketoconazole
In a placebo-controlled, crossover trial in 8 healthy adult volunteers, coadministration of a single dose of orally inhaled fluticasone propionate (1,000 mcg) with multiple doses of ketoconazole (200 mg) to steady state resulted in increased plasma fluticasone propionate exposure, a reduction in plasma cortisol AUC, and no effect on urinary excretion of cortisol.
Following orally inhaled fluticasone propionate alone, AUC(2-last) averaged 1.559 ng•h/mL (range: 0.555 to 2.906 ng•h/mL) and AUC(2-∞) averaged 2.269 ng•h/mL (range: 0.836 to 3.707 ng•h/mL). Fluticasone propionate AUC(2-last) and AUC(2-∞) increased to 2.781 ng•h/mL (range: 2.489 to 8.486 ng•h/mL) and 4.317 ng•h/mL (range: 3.256 to 9.408 ng•h/mL), respectively, after coadministration of ketoconazole with orally inhaled fluticasone propionate. This increase in plasma fluticasone propionate concentration resulted in a decrease (45%) in serum cortisol AUC.
Erythromycin
In a multiple-dose drug interaction trial, coadministration of orally inhaled fluticasone propionate (500 mcg twice daily) and erythromycin (333 mg 3 times daily) did not affect fluticasone propionate pharmacokinetics.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Fluticasone propionate demonstrated no tumorigenic potential in mice at oral doses up to 1,000 mcg/kg (approximately 2 and 10 times the MRHDID for adults and children aged 4 to 11 years, respectively, on a mcg/m² basis) for 78 weeks or in rats at inhalation doses up to 57 mcg/kg (approximately 0.2 times and approximately equivalent to the MRHDID for adults and children aged 4 to 11 years, respectively, on a mcg/m² basis) for 104 weeks.
Fluticasone propionate did not induce gene mutation in prokaryotic or eukaryotic cells in vitro. No significant clastogenic effect was seen in cultured human peripheral lymphocytes in vitro or in the in vivo mouse micronucleus test.
Fertility and reproductive performance were unaffected in male and female rats at subcutaneous doses up to 50 mcg/kg (approximately 0.2 times the MRHDID for adults on a mcg/m² basis).
14. Clinical Studies
14.1 Adult and Adolescent Subjects Aged 12 Years and Older
Four randomized, double-blind, parallel-group, placebo-controlled, U.S. clinical trials were conducted in 1,036 adult and adolescent subjects (aged 12 years and older) with asthma to assess the efficacy and safety of FLOVENT DISKUS in the treatment of asthma. Fixed dosages of 100, 250, and 500 mcg twice daily were compared with placebo to provide information about appropriate dosing to cover a range of asthma severity. Subjects in these trials included those inadequately controlled with bronchodilators alone and those already maintained on daily ICS. All doses were delivered by inhalation of the contents of 1 or 2 blisters from FLOVENT DISKUS twice daily.
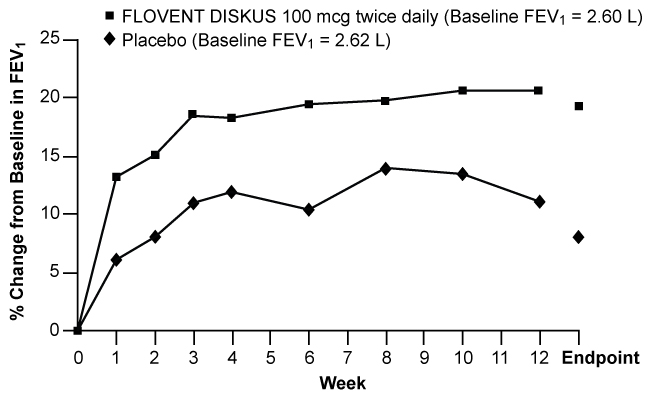
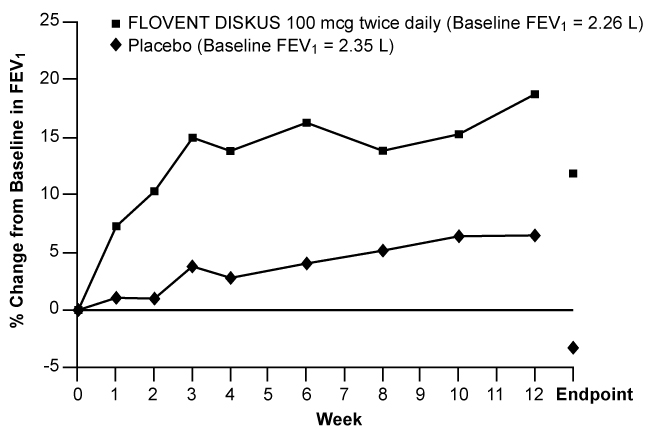
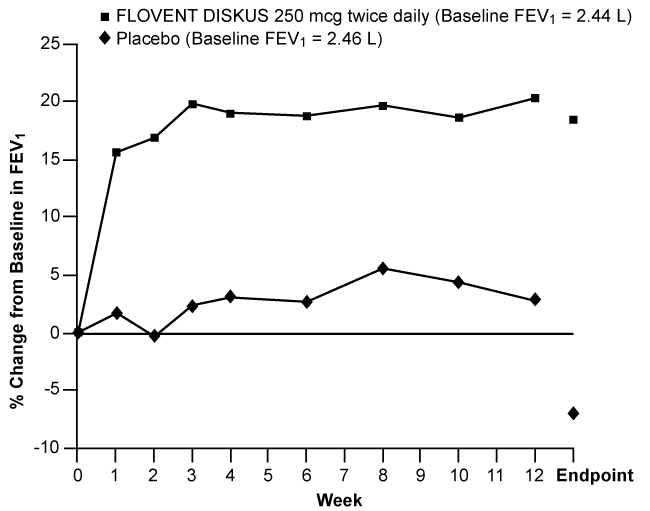
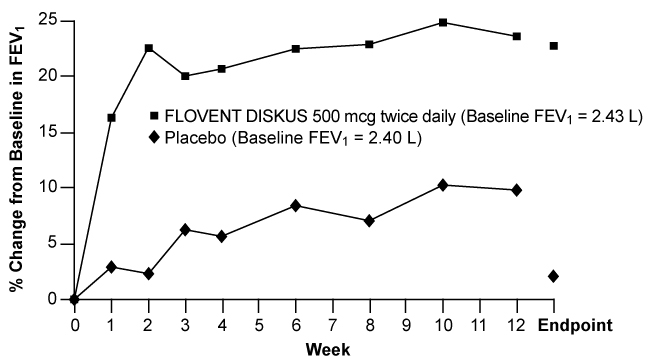
Figures 1 through 4 display results of pulmonary function tests (mean percent change from baseline in FEV1 prior to AM dose) for 3 recommended dosages of FLOVENT DISKUS (100, 250, and 500 mcg twice daily) and placebo from the four 12-week trials in adolescents and adults. These trials used predetermined criteria for lack of efficacy (indicators of worsening asthma), resulting in withdrawal of more patients in the placebo group. Therefore, pulmonary function results at Endpoint (the last evaluable FEV1 result, including most patients' lung function data) are also displayed. Pulmonary function, as determined by percent change from baseline in FEV1 at recommended dosages of FLOVENT DISKUS improved significantly compared with placebo by the first week of treatment, and improvement was maintained for up to 1 year or more.
Figure 1. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 100 mcg Twice Daily in Adults and Adolescents Receiving Bronchodilators Alone:
Figure 2. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 100 mcg Twice Daily in Adults and Adolescents Receiving Inhaled Corticosteroids:
Figure 3. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 250 mcg Twice Daily in Adults and Adolescents Receiving Inhaled Corticosteroids or Bronchodilators Alone:
Figure 4. A 12-Week Clinical Trial Evaluating FLOVENT DISKUS 500 mcg Twice Daily in Adults and Adolescents Receiving Inhaled Corticosteroids or Bronchodilators Alone:
In all 4 efficacy trials, measures of pulmonary function (FEV1) were statistically significantly improved as compared with placebo at all twice-daily doses. Subjects on all dosages of FLOVENT DISKUS were also less likely to discontinue study participation due to asthma deterioration (as defined by predetermined criteria for lack of efficacy including lung function and subject-recorded variables such as AM PEF, albuterol use, and nighttime awakenings due to asthma) compared with placebo.
In a clinical trial of 111 subjects with severe asthma requiring chronic oral prednisone therapy (average baseline daily prednisone dose was 14 mg), fluticasone propionate given by inhalation powder at doses of 500 and 1,000 mcg twice daily was evaluated. Both doses enabled a statistically significantly larger percentage of subjects to wean from oral prednisone as compared with placebo (75% of the subjects on 500 mcg twice daily and 89% of the subjects on 1,000 mcg twice daily as compared with 9% of subjects on placebo). Accompanying the reduction in oral corticosteroid use, subjects treated with fluticasone propionate had significantly improved lung function and fewer asthma symptoms as compared with the placebo group.
14.2 Pediatric Subjects Aged 4 to 11 Years
A 12-week, placebo-controlled clinical trial was conducted in 437 pediatric subjects (177 received FLOVENT DISKUS), approximately half of whom were receiving ICS at baseline. In this trial, doses of fluticasone propionate inhalation powder 50 and 100 mcg twice daily significantly improved FEV1 (15% and 18% change from baseline at Endpoint, respectively) compared with placebo (7% change). AM PEF was also significantly improved with doses of fluticasone propionate 50 and 100 mcg twice daily (26% and 27% change from baseline at Endpoint, respectively) compared with placebo (14% change). In this trial, subjects on active treatment were significantly less likely to discontinue treatment due to asthma deterioration (as defined by predetermined criteria for lack of efficacy including lung function and subject-recorded variables such as AM PEF, albuterol use, and nighttime awakenings due to asthma).
Two other 12-week placebo-controlled clinical trials were conducted in 504 pediatric subjects with asthma, approximately half of whom were receiving ICS at baseline. In these trials, FLOVENT DISKUS was efficacious at doses of 50 and 100 mcg twice daily when compared with placebo on major endpoints including lung function and symptom scores. Pulmonary function improved significantly compared with placebo by the first week of treatment, and subjects treated with FLOVENT DISKUS were also less likely to discontinue trial participation due to asthma deterioration. One hundred ninety-two (192) subjects received FLOVENT DISKUS for up to 1 year during an open-label extension. Data from this open-label extension suggested that lung function improvements could be maintained up to 1 year.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.