FLUORESCITE Solution for injection Ref.[10815] Active ingredients: Fluorescein
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
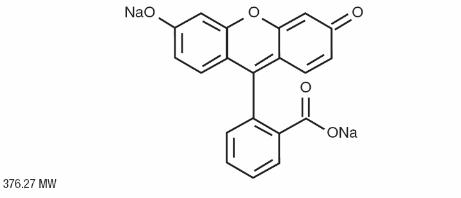
FLUORESCITE (fluorescein injection, USP) 10% contains fluorescein sodium (equivalent to fluorescein 10% w/v). It is a sterile solution for use intravenously as a diagnostic aid. Its chemical name is spiro[isobenzofuran-1(3H),9'-[9H]xanthene]-3-one, 3'6'-dihydroxy, disodium salt.
The active ingredient is represented by the chemical structure:
FLUORESCITE (fluorescein injection, USP) 10% is supplied as a sterile, unpreserved, unit dose aqueous solution, that has a pH of 8.0–9.8 and an osmolality of 572-858 mOsm/kg.
Active ingredient: fluorescein sodium
Inactive Ingredients: Sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection.
| Dosage Forms and Strengths |
|---|
|
Single use 5 mL vial containing 100 mg/mL fluorescein. |
| How Supplied |
|---|
|
FLUORESCITE (fluorescein injection, USP) 10% is supplied in a single use 5 mL glass vial with a gray FluroTec coated chlorobutyl stopper and purple flip-off aluminum seal. The vial stopper is not made with natural rubber latex. The vial contains a sterile, red-orange solution of fluorescein sodium. NDC 0065-0092-05 Distributed By: ALCON LABORATORIES, INC., Fort Worth, Texas 76134 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| FLUORESCITE | Canada, Estonia, Hong Kong, Japan, New Zealand, Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.