FLUVASTATIN Capsules Ref.[8165] Active ingredients: Fluvastatin
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: Sandoz Limited, Frimley Business Park, Frimley, Camberley, Surrey, GU16 7SR
Pharmacodynamic properties
Pharmacotherapeutic group: HMG-CoA reductase inhibitors
ATC code: C10AA04
Fluvastatin, a fully synthetic cholesterol-lowering agent, is a competitive inhibitor of HMG-CoA reductase, which is responsible for the conversion of HMG-CoA to mevalonate, a precursor of sterols, including cholesterol. Fluvastatin exerts its main effect in the liver and is mainly a racemate of the two erythro enantiomers of which one exerts the pharmacological activity. The inhibition of cholesterol biosynthesis reduces the cholesterol in hepatic cells, which stimulates the synthesis of LDL receptors and thereby increases the uptake of LDL particles. The ultimate result of these mechanisms is a reduction in the plasma cholesterol concentration.
Fluvastatin reduces total-C, LDL-C, Apo B, and triglycerides, and increases HDL-C in patients with hypercholesterolaemia and mixed dyslipidaemia.
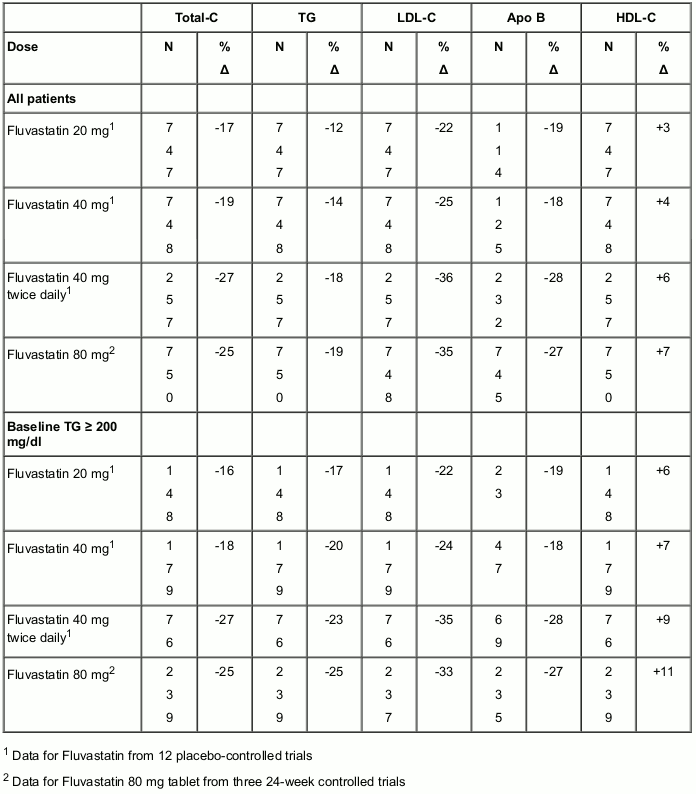
In 12 placebo-controlled studies in patients with Type IIa or IIb hyperlipoproteinaemia, fluvastatin alone was administered to 1,621 patients in daily dose regimens of 20 mg, 40 mg and 80 mg (40 mg twice daily) for at least 6 weeks duration. In a 24-week analysis, daily doses of 20 mg, 40 mg and 80 mg produced dose-related reductions in total-C, LDL-C, Apo B and in triglycerides and increases in HDL-C (see Table 2).
Fluvastatin 80 mg prolonged-release tablets were administered to over 800 patients in three pivotal trials of 24 weeks active treatment duration and compared to fluvastatin 40 mg once or twice daily. Given as a single daily dose of 80 mg, fluvastatin significantly reduced total-C, LDL-C, triglycerides (TG) and Apo B (see Table 2).
Therapeutic response is well established within two weeks, and a maximum response is achieved within four weeks. After four weeks of therapy, the median decrease in LDL-C was 38% and at week 24 (endpoint) the median LDL-C decrease was 35%. Significant increases in HDL-C were also observed.
Table 2. Median percent change in lipid parameters from baseline to week 24 Placebo-controlled studies (fluvastatin immediate-release capsules) and active-controlled trials (fluvastatin prolonged-release tablets):
In the Lipoprotein and Coronary Atherosclerosis Study (LCAS), the effect of fluvastatin on coronary atherosclerosis was assessed by quantitative coronary angiography in male and female patients (35 to 75 years old) with coronary artery disease and baseline LDL-C levels of 3.0 to 4.9 mmol/l (115 to 190 mg/dl). In this randomised, double-blind, controlled clinical study, 429 patients were treated with either fluvastatin 40 mg/day or placebo. Quantitative coronary angiograms were evaluated at baseline and after 2.5 years of treatment and were evaluable in 340 out of 429 patients. Fluvastatin treatment slowed the progression of coronary atherosclerosis lesions by 0.072 mm (95% confidence intervals for treatment difference from −0.1222 to −0.022 mm) over 2.5 years as measured by change in minimum lumen diameter (fluvastatin −0.028 mm vs. placebo −0.100 mm). No direct correlation between the angiographic findings and the risk of cardiovascular events has been demonstrated.
In the Lescol Intervention Prevention Study (LIPS), the effect of fluvastatin on major adverse cardiac events (MACE; i.e. cardiac death, non-fatal myocardial infarction and coronary revascularisation) was assessed in patients with coronary heart disease who had first successful percutaneous coronary intervention. The study included male and female patients (18 to 80 years old) and with baseline total C levels ranging from 3.5 to 7.0 mmol/l (135 to 270 mg/dl).
In this randomised, double-blind, placebo-controlled trial fluvastatin (n=844), given as 80 mg daily over 4 years, significantly reduced the risk of the first MACE by 22% (p=0.013) as compared to placebo (n=833).
The primary endpoint of MACE occurred in 21.4% of patients treated with fluvastatin vs 26.7% of patients treated with placebo (absolute risk difference: 5.2%; 95% CI: 1.1 to 9.3).
These beneficial effects were particularly noteworthy in patients with diabetes mellitus and in patients with multivessel disease.
Paediatric population
Children and adolescents with heterozygous familial hypercholesterolaemia
The safety and efficacy of fluvastatin in children and adolescent patients aged 9-16 years of age with heterozygous familial hypercholesterolaemia has been evaluated in 2 open label, uncontrolled clinical trials of 2 years' duration. 114 patients (66 boys and 48 girls) were treated with fluvastatin administered as either fluvastatin 20 mg/day to 40 mg twice daily or fluvastatin 80 mg prolonged-release tablets once daily using a dose-titration regimen based upon LDL-C response.
The first study enrolled 29 pre-pubertal boys, 9-12 years of age, who had an LDL-C level >90th percentile for age and one parent with primary hypercholesterolaemia and either a family history of premature ischaemic heart disease or tendon xanthomas. The mean baseline LDL-C was 226 mg/dL equivalent to 5.8 mmol/L (range: 137-354 mg/dL equivalent to 3.6-9.2 mmol/L). All patients were started on fluvastatin 20 mg daily with dose adjustments every 6 weeks to 40 mg daily then 80 mg daily (40 mg twice daily) to achieve an LDL-C goal of 96.7 to 123.7 mg/dL (2.5 mmol/L to 3.2 mmol/L).
The second study enrolled 85 male and female patients, 10 to 16 years of age, who had an LDL-C >190 mg/dL (equivalent to 4.9 mmol/L) or LDL-C >160 mg/dL (equivalent to 4.1 mmol/L) and one or more risk factors for coronary heart disease, or LDL-C >160 mg/dL (equivalent to 4.1 mmol/L) and a proven LDL-receptor defect. The mean baseline LDL-C was 225 mg/dL equivalent to 5.8 mmol/L (range: 148-343 mg/dL equivalent to 3.8-8.9 mmol/L). All patients were started on fluvastatin 20 mg capsules daily with dose adjustments every 6 weeks to 40 mg daily then 80 mg fluvastatin prolonged-release tablets daily to achieve an LDL-C goal of <130 mg/dL (3.4 mmol/L). 70 patients were pubertal or postpubertal (n=69 evaluated for efficacy).
In the first study (in prepubertal boys), fluvastatin 20 to 80 mg daily doses decreased plasma levels of total-C and LDL-C by 21% and 27%, respectively. The mean achieved LDL-C was 161 mg/dL equivalent to 4.2 mmol/L (range: 74-336 mg/dL equivalent 1.9- 8.7 mmol/L). In the second study (in pubertal and postpubertal girls and boys), fluvastatin 20 to 80 mg daily doses decreased plasma levels of total-C and LDL-C by 22% and 28%, respectively. The mean achieved LDL-C was 159 mg/dL equivalent to 4.1 mmol/L (range: 90-295 mg/dL equivalent to 2.3-7.6 mmol/L).
The majority of patients in both studies (83% in the first study and 89% in the second study) were titrated to the maximum daily dose of 80 mg. At study endpoint, 26 to 30% of patients in both studies achieved a targeted LDL-C goal of <130 mg/dL (3.4 mmol/L).
Pharmacokinetic properties
Absorption
Fluvastatin is absorbed rapidly and completely (98%) after oral administration of a solution to fasted volunteers. After oral administration of fluvastatin 80 mg prolonged-release tablets, and in comparison with the immediate-release capsules, the absorption rate of fluvastatin is almost 60% slower while the mean residence time of fluvastatin is increased by approximately 4 hours. In a fed state, the substance is absorbed at a reduced rate.
Distribution
Fluvastatin exerts its main effect in the liver, which is also the main organ for its metabolism. The absolute bioavailability assessed from systemic blood concentrations is 24%. The apparent volume of distribution (Vz/f) for the drug is 330 litres. More than 98% of the circulating drug is bound to plasma proteins, and this binding is not affected either by the concentration of fluvastatin, or by warfarin, salicylic acid or glyburide.
Biotransformation
Fluvastatin is mainly metabolised in the liver. The major components circulating in the blood are fluvastatin and the pharmacologically inactive N-desisopropyl-propionic acid metabolite. The hydroxylated metabolites have pharmacological activity but do not circulate systemically. There are multiple, alternative cytochrome P450 (CYP450) pathways for fluvastatin biotransformation and thus fluvastatin metabolism is relatively insensitive to CYP450 inhibition.
Fluvastatin inhibited only the metabolism of compounds that are metabolised by CYP2C9. Despite the potential that therefore exists for competitive interaction between fluvastatin and compounds that are CYP2C9 substrates, such as diclofenac, phenytoin, tolbutamide, and warfarin, clinical data indicate that this interaction is unlikely.
Elimination
Following administration of 3H-fluvastatin to healthy volunteers, excretion of radioactivity is about 6% in the urine and 93% in the faeces, and fluvastatin accounts for less than 2% of the total radioactivity excreted. The plasma clearance (CL/f) for fluvastatin in man is calculated to be 1.8 ± 0.8 L/min. Steady-state plasma concentrations show no evidence of fluvastatin accumulation following administration of 80 mg daily. Following oral administration of 40 mg fluvastatin, the terminal disposition half-life for fluvastatin is 2.3 ± 0.9 hours.
Characteristics in patients
Plasma concentrations of fluvastatin do not vary as a function of either age or gender in the general population. However, enhanced treatment response was observed in women and in elderly people.
Since fluvastatin is eliminated primarily via the biliary route and is subject to significant pre-systemic metabolism, the potential exists for drug accumulation in patients with hepatic insufficiency (see sections 4.3 and 4.4).
Children and adolescents with heterozygous familial hypercholesterolaemia
No pharmacokinetic data in children are available.
Preclinical safety data
The conventional studies, including safety pharmacology, genotoxicity, repeated dose toxicity, carcinogenicity and toxicity on reproduction studies did not indicate other risks for the patient than those expected due to the pharmacological mechanism of action. A variety of changes were identified in toxicity studies that are common to HMG-CoA reductase inhibitors. Based on clinical observations, liver function tests are already recommended (see section 4.4). Further toxicity seen in animals was either not relevant for human use or occurred at exposure levels sufficiently in excess of the maximum human exposure indicating little relevance to clinical use. Despite the theoretical considerations concerning the role of cholesterol in embryo development, animal studies did not suggest an embryotoxic and teratogenic potential of fluvastatin.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.