FOLOTYN Solution for injection Ref.[10174] Active ingredients: Pralatrexate
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
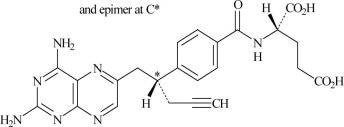
Pralatrexate is a dihydrofolate reductase inhibitor. Pralatrexate has the chemical name (2S)2[[4-[(1RS)1[(2,4-diaminopteridin-6-yl)methyl]but-3-ynyl]benzoyl]amino]pentanedioic acid. The molecular formula is C23H23N7O5 and the molecular weight is 477.48 g/mol. Pralatrexate is a 1:1 racemic mixture of S- and R-diastereomers at the C10 position (indicated with *).
The structural formula is as follows:
Pralatrexate is an off-white to yellow solid. It is soluble in aqueous solutions at pH 6.5 or higher. Pralatrexate is practically insoluble in chloroform and ethanol. The pKa values are 3.25, 4.76, and 6.17.
FOLOTYN (pralatrexate) is supplied as a preservative-free, sterile, isotonic, non-pyrogenic clear yellow aqueous solution contained in a clear glass single-dose vial (Type I) for intravenous use. Each 1 mL of solution contains 20 mg of pralatrexate, sufficient sodium chloride to achieve an isotonic (280-300 mOsm) solution, and sufficient sodium hydroxide, and hydrochloric acid if needed, to adjust and maintain the pH at 7.5-8.5. FOLOTYN is supplied as either 20 mg (1 mL) or 40 mg (2 mL) single-dose vials at a concentration of 20 mg/mL.
| Dosage Forms and Strengths |
|---|
|
Injection: 40 mg/2 mL (20 mg/mL) and 20 mg/mL clear yellow sterile solution in single-dose vial |
| How Supplied |
|---|
|
FOLOTYN is available in clear glass single-dose vials containing pralatrexate at a concentration of 20 mg/mL as a preservative-free, sterile, clear yellow solution individually packaged for intravenous use in the following presentations: NDC 72893-003-01: 20 mg of pralatrexate in 1 mL solution in a vial (20 mg/1 mL) NDC 72893-005-01: 40 mg of pralatrexate in 2 mL solution in a vial (40 mg/2 mL) |
Drugs
| Drug | Countries | |
|---|---|---|
| FOLOTYN | Australia, Canada, Singapore, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.