FORTEO Solution for injection Ref.[10817] Active ingredients: Teriparatide
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Endogenous 84-amino acid parathyroid hormone (PTH) is the primary regulator of calcium and phosphate metabolism in bone and kidney. Physiological actions of PTH include regulation of bone metabolism, renal tubular reabsorption of calcium and phosphate, and intestinal calcium absorption. The biological actions of PTH and teriparatide are mediated through binding to specific high-affinity cell-surface receptors. Teriparatide and the 34 N-terminal amino acids of PTH bind to these receptors with the same affinity and have the same physiological actions on bone and kidney. Teriparatide is not expected to accumulate in bone or other tissues.
The skeletal effects of teriparatide depend upon the pattern of systemic exposure. Once-daily administration of teriparatide stimulates new bone formation on trabecular and cortical (periosteal and/or endosteal) bone surfaces by preferential stimulation of osteoblastic activity over osteoclastic activity. In monkey studies, teriparatide improved trabecular microarchitecture and increased bone mass and strength by stimulating new bone formation in both cancellous and cortical bone. In humans, the anabolic effects of teriparatide manifest as an increase in skeletal mass, an increase in markers of bone formation and resorption, and an increase in bone strength. By contrast, continuous excess of endogenous PTH, as occurs in hyperparathyroidism, may be detrimental to the skeleton because bone resorption may be stimulated more than bone formation.
12.2. Pharmacodynamics
Pharmacodynamics in Men with Primary or Hypogonadal Osteoporosis and Postmenopausal Women with Osteoporosis
Effects on Mineral Metabolism
Teriparatide affects calcium and phosphorus metabolism in a pattern consistent with the known actions of endogenous PTH (e.g., increases serum calcium and decreases serum phosphorus).
Serum Calcium Concentrations
When teriparatide 20 mcg was administered once daily, the serum calcium concentration increased transiently, beginning approximately 2 hours after dosing and reaching a maximum concentration between 4 and 6 hours (median increase, 0.4 mg/dL). The serum calcium concentration began to decline approximately 6 hours after dosing and returned to baseline by 16 to 24 hours after each dose.
In a clinical study of postmenopausal women with osteoporosis, the median peak serum calcium concentration measured 4 to 6 hours after dosing with FORTEO (20 mcg subcutaneous once daily) was 9.68 mg/dL at 12 months. The peak serum calcium remained below 11 mg/dL in >99% of women at each visit. Sustained hypercalcemia was not observed.
In this study, 11.1% of women treated with FORTEO had at least 1 serum calcium value above the upper limit of normal (ULN) (10.6 mg/dL) compared with 1.5% of women treated with placebo. The percentage of women treated with FORTEO whose serum calcium was above the ULN on consecutive 4- to 6-hour post-dose measurements was 3% compared with 0.2% of women treated with placebo. In these women, calcium supplements and/or FORTEO doses were reduced. The timing of these dose reductions was at the discretion of the investigator. FORTEO dose adjustments were made at varying intervals after the first observation of increased serum calcium (median 21 weeks). During these intervals, there was no evidence of progressive increases in serum calcium.
In a clinical study of men with either primary or hypogonadal osteoporosis, the effects on serum calcium were similar to those observed in postmenopausal women. The median peak serum calcium concentration measured 4 to 6 hours after dosing with FORTEO was 9.44 mg/dL at 12 months. The peak serum calcium remained below 11 mg/dL in 98% of men at each visit. Sustained hypercalcemia was not observed.
In this study, 6% of men treated with FORTEO daily had at least 1 serum calcium value above the ULN (10.6 mg/dL) compared with none of the men treated with placebo. The percentage of men treated with FORTEO whose serum calcium was above the ULN on consecutive measurements was 1.3% (2 men) compared with none of the men treated with placebo. Calcium supplementation was reduced in these men [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
In a clinical study of women previously treated for 18 to 39 months with raloxifene (n=26) or alendronate (n=33), mean serum calcium >12 hours after FORTEO treatment was increased by 0.36 to 0.56 mg/dL, after 1 to 6 months of FORTEO treatment compared with baseline. Of the women pretreated with raloxifene, 3 (11.5%) had a serum calcium >11 mg/dL, and of those pretreated with alendronate, 3 (9.1%) had a serum calcium >11 mg/dL. The highest serum calcium reported was 12.5 mg/dL. None of the women had symptoms of hypercalcemia. There were no placebo controls in this study.
In the study of patients with glucocorticoid-induced osteoporosis, the effects of FORTEO on serum calcium were similar to those observed in postmenopausal women with osteoporosis not taking glucocorticoids.
Urinary Calcium Excretion
In a clinical study of postmenopausal women with osteoporosis who received 1000 mg of supplemental calcium and at least 400 IU of vitamin D, daily FORTEO increased urinary calcium excretion. The median urinary excretion of calcium was 190 mg/day at 6 months and 170 mg/day at 12 months. These levels were 30 mg/day and 12 mg/day higher, respectively, than in women treated with placebo. The incidence of hypercalciuria (>300 mg/day) was similar in the women treated with FORTEO or placebo.
In a clinical study of men with either primary or hypogonadal osteoporosis who received 1000 mg of supplemental calcium and at least 400 IU of vitamin D, daily FORTEO had inconsistent effects on urinary calcium excretion. The median urinary excretion of calcium was 220 mg/day at 1 month and 210 mg/day at 6 months. These levels were 20 mg/day higher and 8 mg/day lower, respectively, than in men treated with placebo. The incidence of hypercalciuria (>300 mg/day) was similar in the men treated with FORTEO or placebo.
Phosphorus and Vitamin D
In single-dose studies, teriparatide produced transient phosphaturia and mild transient reductions in serum phosphorus concentration. However, hypophosphatemia (<2.4 mg/dL) was not observed in clinical trials with FORTEO.
In clinical trials of daily FORTEO, the median serum concentration of 1,25-dihydroxyvitamin D was increased at 12 months by 19% in women and 14% in men, compared with baseline. In the placebo group, this concentration decreased by 2% in women and increased by 5% in men. The median serum 25-hydroxyvitamin D concentration at 12 months was decreased by 19% in women and 10% in men compared with baseline. In the placebo group, this concentration was unchanged in women and increased by 1% in men.
In the study of patients with glucocorticoid-induced osteoporosis, the effects of FORTEO on serum phosphorus were similar to those observed in postmenopausal women with osteoporosis not taking glucocorticoids.
Effects on Markers of Bone Turnover
Daily administration of FORTEO to men and postmenopausal women with osteoporosis in clinical studies stimulated bone formation, as shown by increases in the formation markers serum bone-specific alkaline phosphatase (BSAP) and procollagen I carboxy-terminal propeptide (PICP). Data on biochemical markers of bone turnover were available for the first 12 months of treatment. Peak concentrations of PICP at 1 month of treatment were approximately 41% above baseline, followed by a decline to near-baseline values by 12 months. BSAP concentrations increased by 1 month of treatment and continued to rise more slowly from 6 through 12 months. The maximum increases of BSAP were 45% above baseline in women and 23% in men. After discontinuation of therapy, BSAP concentrations returned toward baseline. The increases in formation markers were accompanied by secondary increases in the markers of bone resorption: urinary N-telopeptide (NTX) and urinary deoxypyridinoline (DPD), consistent with the physiological coupling of bone formation and resorption in skeletal remodeling. Changes in BSAP, NTX, and DPD were lower in men than in women, possibly because of lower systemic exposure to teriparatide in men.
In the study of patients with glucocorticoid-induced osteoporosis, the effects of FORTEO on serum markers of bone turnover were similar to those observed in postmenopausal women with osteoporosis not taking glucocorticoids.
12.3. Pharmacokinetics
Absorption
Teriparatide is absorbed after subcutaneous injection; the absolute bioavailability is approximately 95% based on pooled data from 20-, 40-, and 80-mcg doses (1-, 2-, and 4-times the recommended dosage, respectively). The peptide reaches peak serum concentrations about 30 minutes after subcutaneous injection of a 20-mcg dose and declines to non-quantifiable concentrations within 3 hours.
Distribution
Volume of distribution following intravenous injection is approximately 0.12 L/kg..
Elimination
Systemic clearance of teriparatide (approximately 62 L/hour in women and 94 L/hour in men) exceeds the rate of normal liver plasma flow, consistent with both hepatic and extra-hepatic clearance. The half-life of teriparatide in serum was approximately 1 hour when administered by subcutaneous injection.
No metabolism or excretion studies have been performed with teriparatide. Peripheral metabolism of PTH is believed to occur by non-specific enzymatic mechanisms in the liver followed by excretion via the kidneys.
Specific Populations
Geriatric Patients
No age-related differences in teriparatide pharmacokinetics were detected (range 31 to 85 years).
Male and Female Patients
Although systemic exposure to teriparatide was approximately 20% to 30% lower in men than women, the recommended dosage for men and women is the same.
Racial Groups
The influence of race has not been determined.
Patients with Renal Impairment
No pharmacokinetic differences were identified in 11 patients with creatinine clearance (CrCl) 30 to 72 mL/minute administered a single dose of teriparatide. In 5 patients with severe renal impairment (CrCl<30 mL/minute), the AUC and T1/2 of teriparatide were increased by 73% and 77%, respectively. Maximum serum concentration of teriparatide was not increased. No studies have been performed in patients undergoing dialysis for chronic renal failure.
Patients with Hepatic Impairment
No studies have been performed in patients with hepatic impairment. Non-specific proteolytic enzymes in the liver (possibly Kupffer cells) cleave PTH and PTH into fragments that are cleared from the circulation mainly by the kidney.
Drug Interaction Studies
Digoxin
In a study of 15 healthy people administered digoxin daily to steady state, a single FORTEO dose did not alter the effect of digoxin on the systolic time interval (from electrocardiographic Q-wave onset to aortic valve closure, a measure of digoxin’s calcium-mediated cardiac effect).
Hydrochlorothiazide
In a study of 20 healthy people, the coadministration of hydrochlorothiazide 25 mg with 40 mcg of FORTEO (2 times the recommended dose) did not affect the serum calcium response to FORTEO. The 24-hour urine excretion of calcium was reduced by a clinically unimportant amount (15%). The effect of coadministration of a higher dose of hydrochlorothiazide with FORTEO on serum calcium levels has not been studied.
Furosemide
In a study of 9 healthy people and 17 patients with CrCl 13 to 72 mL/minute, coadministration of intravenous furosemide (20 to 100 mg) with FORTEO 40 mcg (2 times the recommended dose) resulted in small increases in the serum calcium (2%) and 24-hour urine calcium (37%); however, these changes did not appear to be clinically important.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Two carcinogenicity bioassays were conducted in Fischer 344 rats. In the first study, male and female rats were given daily subcutaneous teriparatide injections of 5, 30, or 75 mcg/kg/day for 24 months from 2 months of age. These doses resulted in rat systemic exposures that were 3, 20, and 60 times higher than the systemic exposure observed in humans, respectively, following a subcutaneous dose of 20 mcg (based on AUC comparison). Teriparatide treatment resulted in a marked dose-related increase in the incidence of osteosarcoma, a rare malignant bone tumor, in both male and female rats. Osteosarcomas were observed at all doses and the incidence reached 40% to 50% in the high-dose groups. Teriparatide also caused a dose-related increase in osteoblastoma and osteoma in both sexes. No osteosarcomas, osteoblastomas or osteomas were observed in untreated control rats. The bone tumors in rats occurred in association with a large increase in bone mass and focal osteoblast hyperplasia.
The second 2-year study was carried out in order to determine the effect of treatment duration and animal age on the development of bone tumors. Female rats were treated for different periods between 2 and 26 months of age with subcutaneous teriparatide doses of 5 and 30 mcg/kg (equivalent to 3 and 20 times the human exposure at the 20-mcg dose, respectively, based on AUC comparison). The study showed that the occurrence of osteosarcoma, osteoblastoma and osteoma was dependent upon dose and duration of teriparatide exposure. Bone tumors were observed when immature 2-month old rats were treated with 30 mcg/kg/day of teriparatide for 24 months or with 5 or 30 mcg/kg/day of teriparatide for 6 months. Bone tumors were also observed when mature 6-month old rats were treated with 30 mcg/kg/day of teriparatide for 6 or 20 months. Tumors were not detected when mature 6-month old rats were treated with 5 mcg/kg/day of teriparatide for 6 or 20 months. The results did not demonstrate a difference in susceptibility to bone tumor formation, associated with teriparatide treatment, between mature and immature rats.
No bone tumors were detected in a long-term monkey study [see Nonclinical Toxicology (13.2)].
Mutagenesis
Teriparatide was not genotoxic in any of the following test systems: the Ames test for bacterial mutagenesis; the mouse lymphoma assay for mammalian cell mutation; the chromosomal aberration assay in Chinese hamster ovary cells, with and without metabolic activation; and the in vivo micronucleus test in mice.
Impairment of Fertility
No effects on fertility were observed in male and female rats given subcutaneous teriparatide doses of 30, 100, or 300 mcg/kg/day prior to mating and in females continuing through gestation Day 6 (16 to 160 times the human dose of 20 mcg based on surface area, mcg/m²).
13.2. Animal Toxicology and/or Pharmacology
In single-dose rodent studies using subcutaneous injection of teriparatide, no mortality was seen in rats given doses of 1000 mcg/kg (540 times the human dose based on surface area, mcg/m²) or in mice given 10,000 mcg/kg (2700 times the human dose based on surface area, mcg/m²).
In a long-term study, skeletally mature ovariectomized female monkeys (N=30 per treatment group) were given either daily subcutaneous teriparatide injections of 5 mcg/kg or vehicle. Following the 18-month treatment period, the monkeys were removed from teriparatide treatment and were observed for an additional 3 years. The 5 mcg/kg dose resulted in systemic exposures that were approximately 6 times higher than the systemic exposure observed in humans following a subcutaneous dose of 20 mcg (based on AUC comparison). Bone tumors were not detected by radiographic or histologic evaluation in any monkey in the study.
14. Clinical Studies
14.1 Treatment of Osteoporosis in Postmenopausal Women
The safety and efficacy of once-daily FORTEO, median exposure of 19 months, were examined in a double-blind, multicenter, placebo-controlled clinical study of 1637 postmenopausal women with osteoporosis. In this study 541 postmenopausal women were treated with 20 mcg FORTEO subcutaneously once daily.
All women received 1000 mg of calcium and at least 400 IU of vitamin D per day. Baseline and endpoint spinal radiographs were evaluated using the semiquantitative scoring. Ninety percent of the women in the study had 1 or more radiographically diagnosed vertebral fractures at baseline. The primary efficacy endpoint was the occurrence of new radiographically diagnosed vertebral fractures defined as changes in the height of previously undeformed vertebrae. Such fractures are not necessarily symptomatic.
Effect on Fracture Incidence
New Vertebral Fractures
FORTEO, when taken with calcium and vitamin D and compared with calcium and vitamin D alone, reduced the risk of 1 or more new vertebral fractures from 14.3% of women in the placebo group to 5.0% in the FORTEO group (444 of the 541 patients treated with 20 mcg once daily of FORTEO were included in this analysis). This difference was statistically significant (p<0.001); the absolute reduction in risk was 9.3% and the relative reduction was 65%. FORTEO was effective in reducing the risk for vertebral fractures regardless of age, baseline rate of bone turnover, or baseline BMD (see Table 2).
Table 2. Effect of FORTEO on Risk of Vertebral Fractures in Postmenopausal Women with Osteoporosis:
| Percent of Women With Fracture | ||||
|---|---|---|---|---|
| FORTEO (N=444) | Placebo (N=448) | Absolute Risk Reduction (, 95 CI) | Relative Risk Reduction (, 95 CI) | |
| New fracture (≥1) | 5.0a | 14.3 | 9.3 (5.5-13.1) | 65 (45-78) |
| 1 fracture | 3.8 | 9.4 | ||
| 2 fractures | 0.9 | 2.9 | ||
| ≥3 fractures | 0.2 | 2.0 | ||
a p≤0.001 compared with placebo.
New Nonvertebral Osteoporotic Fractures
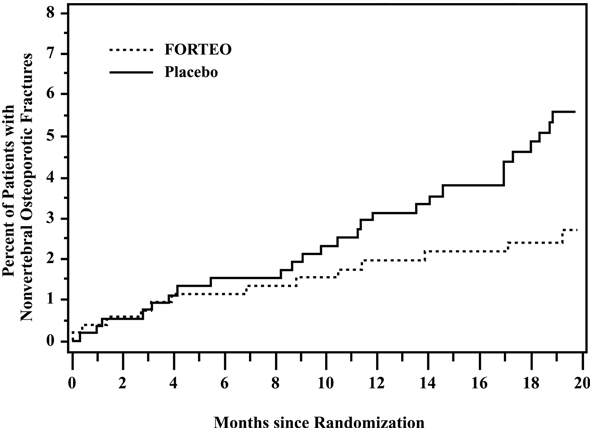
FORTEO significantly reduced the risk of any nonvertebral fracture from 5.5% in the placebo group to 2.6% in the FORTEO group (p<0.05). The absolute reduction in risk was 2.9% and the relative reduction was 53%. The incidence of new nonvertebral fractures in the FORTEO group compared with the placebo group was ankle/foot (0.2%, 0.7%), hip (0.2%, 0.7%), humerus (0.4%, 0.4%), pelvis (0%, 0.6%), ribs (0.6%, 0.9%), wrist (0.4%, 1.3%), and other sites (1.1%, 1.5%), respectively.
The cumulative percentage of postmenopausal women with osteoporosis who sustained new nonvertebral fractures was lower in women treated with FORTEO than in women treated with placebo (see Figure 1).
Figure 1. Cumulative Percentage of Postmenopausal Women with Osteoporosis Sustaining New Nonvertebral Osteoporotic Fractures:
Effect on Bone Mineral Density (BMD)
FORTEO increased lumbar spine BMD in postmenopausal women with osteoporosis. Statistically significant increases were seen at 3 months and continued throughout the treatment period. Postmenopausal women with osteoporosis who were treated with FORTEO had statistically significant increases in BMD from baseline to endpoint at the lumbar spine, femoral neck, total hip, and total body (see Table 3).
Table 3. Mean Percent Change in BMD from Baseline to Endpointa in Postmenopausal Women with Osteoporosis, Treated with FORTEO or Placebo for a Median of 19 Months:
| FORTEO N=541 | Placebo N=544 | |
|---|---|---|
| Lumbar spine BMD | 9.7b | 1.1 |
| Femoral neck BMD | 2.8c | -0.7 |
| Total hip BMD | 2.6c | -1.0 |
| Trochanter BMD | 3.5c | -0.2 |
| Intertrochanter BMD | 2.6c | -1.3 |
| Ward’s triangle BMD | 4.2c | -0.8 |
| Total body BMD | 0.6c | -0.5 |
| Distal ⅓ radius BMD | -2.1 | -1.3 |
| Ultradistal radius BMD | -0.1 | -1.6 |
a Intent-to-treat analysis, last observation carried forward.
b p<0.001 compared with placebo.
c p<0.05 compared with placebo.
FORTEO treatment increased lumbar spine BMD from baseline in 96% of postmenopausal women treated. Seventy-two percent of patients treated with FORTEO achieved at least a 5% increase in spine BMD, and 44% gained 10% or more.
Both treatment groups lost height during the trial. The mean decreases were 3.61 and 2.81 mm in the placebo and FORTEO groups, respectively.
Bone Histology
The effects of FORTEO on bone histology were evaluated in iliac crest biopsies of 35 postmenopausal women treated for 12 to 24 months with calcium and vitamin D and FORTEO. Normal mineralization was observed with no evidence of cellular toxicity. The new bone formed with FORTEO was of normal quality (as evidenced by the absence of woven bone and marrow fibrosis).
14.2 Treatment to Increase Bone Mass in Men with Primary or Hypogonadal Osteoporosis
The safety and efficacy of once-daily FORTEO, median exposure of 10 months, were examined in a double-blind, multicenter, placebo-controlled clinical study of 437 men with either primary (idiopathic) or hypogonadal osteoporosis. In this study, 151 men received 20 mcg of FORTEO given subcutaneously once daily. All men received 1000 mg of calcium and at least 400 IU of vitamin D per day. The primary efficacy endpoint was change in lumbar spine BMD.
FORTEO increased lumbar spine BMD in men with primary or hypogonadal osteoporosis. Statistically significant increases were seen at 3 months and continued throughout the treatment period. FORTEO was effective in increasing lumbar spine BMD regardless of age, baseline rate of bone turnover, and baseline BMD. The effects of FORTEO at additional skeletal sites are shown in Table 4.
FORTEO treatment for a median of 10 months increased lumbar spine BMD from baseline in 94% of men treated. Fifty-three percent of patients treated with FORTEO achieved at least a 5% increase in spine BMD, and 14% gained 10% or more.
Table 4. Mean Percent Change in BMD from Baseline to Endpointa in Men with Primary or Hypogonadal Osteoporosis, Treated with FORTEO or Placebo for a Median of 10 Months:
| FORTEO N=151 | Placebo N=147 | |
|---|---|---|
| Lumbar spine BMD | 5.9b | 0.5 |
| Femoral neck BMD | 1.5c | 0.3 |
| Total hip BMD | 1.2 | 0.5 |
| Trochanter BMD | 1.3 | 1.1 |
| Intertrochanter BMD | 1.2 | 0.6 |
| Ward’s triangle BMD | 2.8 | 1.1 |
| Total body BMD | 0.4 | -0.4 |
| Distal ⅓ radius BMD | -0.5 | -0.2 |
| Ultradistal radius BMD | -0.5 | -0.3 |
a Intent-to-treat analysis, last observation carried forward.
b p<0.001 compared with placebo.
c p<0.05 compared with placebo.
14.3 Treatment of Men and Women with Glucocorticoid-Induced Osteoporosis
The efficacy of FORTEO for treating glucocorticoid-induced osteoporosis was assessed in a randomized, double-blind, active-controlled trial of 428 patients (19% men, 81% women) aged 22 to 89 years (mean 57 years) treated with ≥5 mg/day prednisone or equivalent for a minimum of 3 months. The duration of the trial was 18 months. In the trial 214 patients were treated with FORTEO 20 mcg given subcutaneously once daily. In the FORTEO group, the baseline median glucocorticoid dose was 7.5 mg/day and the baseline median duration of glucocorticoid use was 1.5 years. The mean (SD) baseline lumbar spine BMD was 0.85 ± 0.13 g/cm² and lumbar spine BMD T-score was –2.5 ± 1 (number of standard deviations below the mean BMD value for healthy adults). A total of 30% of patients had prevalent vertebral fracture(s) and 43% had prior non-vertebral fracture(s). The patients had chronic rheumatologic, respiratory or other diseases that required sustained glucocorticoid therapy. All patients received 1000 mg of calcium plus 800 IU of vitamin D supplementation per day.
Because of differences in mechanism of action (anabolic vs. anti-resorptive) and lack of clarity regarding differences in BMD as an adequate predictor of fracture efficacy, data on the active comparator are not presented.
Effect on Bone Mineral Density (BMD)
In patients with glucocorticoid-induced osteoporosis, FORTEO increased lumbar spine BMD compared with baseline at 3 months through 18 months of treatment. In patients treated with FORTEO, the mean percent change in BMD from baseline to endpoint was 7.2% at the lumbar spine, 3.6% at the total hip, and 3.7% at the femoral neck (p<0.001 all sites). The relative treatment effects of FORTEO were consistent in subgroups defined by gender, age, geographic region, body mass index, underlying disease, prevalent vertebral fracture, baseline glucocorticoid dose, prior bisphosphonate use, and glucocorticoid discontinuation during trial.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.