FRUZAQLA Hard capsule Ref.[107236] Active ingredients: Fruquintinib
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
Fruquintinib is a small molecule kinase inhibitor of vascular endothelial growth factor receptors (VEGFR)-1, -2, and -3 with IC50 values of 33, 35, and 0.5 nM, respectively. In vitro studies showed fruquintinib inhibited VEGF-mediated endothelial cell proliferation and tubular formation. In vitro and in vivo studies showed fruquintinib inhibited VEGF-induced VEGFR-2 phosphorylation. In vivo studies showed fruquintinib inhibited tumor growth in a tumor xenograft mouse model of colon cancer.
12.2. Pharmacodynamics
Fruquintinib exposure-response relationships and the time course of pharmacodynamic response are unknown.
Cardiac Electrophysiology
A mean increase in QTc interval >20 milliseconds (ms) was not observed at the approved recommended dosage.
12.3. Pharmacokinetics
The fruquintinib steady-state geometric mean (% coefficient of variation [CV]) maximum concentration (Cmax) is 300 ng/mL (28%) and area under the concentration-time curve for the dosing interval (AUC0-24h) is 5880 ng∙h/mL (29%) at the recommended dosage. The fruquintinib Cmax and AUC0-24h are dose-proportional across the dosage range of 1 to 6 mg (0.2 to 1.2 times the recommended dosage). Fruquintinib steady state is achieved after 14 days with a mean AUC0-24h accumulation of 4-fold.
Absorption
The fruquintinib median (min, max) time to Cmax is approximately 2 hours (0, 26 hours).
Effect of Food
No clinically significant differences in fruquintinib pharmacokinetics were observed following administration of a high-fat meal (800 to 1000 calories, 50% fat).
Distribution
The mean (SD) apparent volume of distribution of fruquintinib is approximately 46 (13) L. Plasma protein binding of fruquintinib is approximately 95%.
Elimination
The fruquintinib mean (SD) elimination half-life is approximately 42 (11) hours and the apparent clearance is 14.8 (4.4) mL/min.
Metabolism
Fruquintinib is primarily eliminated by CYP450 and non-CYP450 (i.e., sulfation and glucuronidation) metabolism. CYP3A and to a lesser extent CYP2C8, CYP2C9, and CYP2C19 are the CYP450 enzymes involved in fruquintinib metabolism.
Excretion
Following oral administration of a 5 mg radiolabeled fruquintinib dose, approximately 60% of the dose was recovered in urine (0.5% unchanged) and 30% of the dose was recovered in feces (5% unchanged).
Specific Populations
No clinically significant differences in the pharmacokinetics of fruquintinib were observed based on age (18 to 82 years), sex, race (Asian, Black, and White), ethnicity (Hispanic/Latino vs. non-Hispanic/Latino), body weight (48 to 108 kg), mild to moderate renal impairment (CrCL 30 to 89 mL/min), mild hepatic impairment (total bilirubin less than or equal to ULN with AST greater than ULN or total bilirubin greater than 1 to 1.5 times ULN with any AST).
The effect of moderate to severe hepatic impairment (total bilirubin greater than 1.5 times ULN and any AST) on fruquintinib pharmacokinetics is unknown.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Strong CYP3A inducers: Fruquintinib Cmax decreased by 12% and AUCinf by 65% following concomitant use with rifampin (strong CYP3A inducer).
Moderate CYP3A inducers: Fruquintinib Cmax is predicted to decrease by 4% and AUCinf by 32% following concomitant use with efavirenz (moderate CYP3A inducer).
Other Drugs: No clinically significant differences in fruquintinib pharmacokinetics were observed when used concomitantly with itraconazole (strong CYP3A inhibitor) or rabeprazole (proton pump inhibitor; gastric acid reducing agent).
No clinically significant differences in the pharmacokinetics of the following drugs were observed when used concomitantly with fruquintinib: dabigatran etexilate (P-gp substrate), or rosuvastatin (BCRP substrate).
In Vitro Studies
Cytochrome P450 Enzymes: Fruquintinib is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A, or an inducer of CYP1A2, CYP2B6, CYP3A.
Transporter Systems: Fruquintinib is not a substrate of P-glycoprotein (P-gp), organic anion transporting polypeptide (OATP)1B1 or OATP1B3. Fruquintinib is not an inhibitor of OATP1B1, OATP1B3, organic anion transporter (OAT)1, OAT3, organic cation transporter (OCT)2, multidrug and toxin extrusion protein (MATE)1, or MATE2-K.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with fruquintinib.
Fruquintinib was not mutagenic in the in vitro bacterial reverse mutation (Ames) assay or clastogenic in the in vitro Chinese hamster ovary chromosome aberration assay. Fruquintinib was not genotoxic in the in vivo rat micronucleus or alkaline comet assays.
13.2. Animal Toxicology and/or Pharmacology
In repeat dose toxicity studies in rats, daily oral administration of fruquintinib at doses ≥0.6 mg/kg (approximately 1.2 times the recommended clinical dose of 5 mg based on BSA) resulted in broken or lost teeth.
14. Clinical Studies
14.1. Metastatic Colorectal Cancer
FRESCO-2 Study
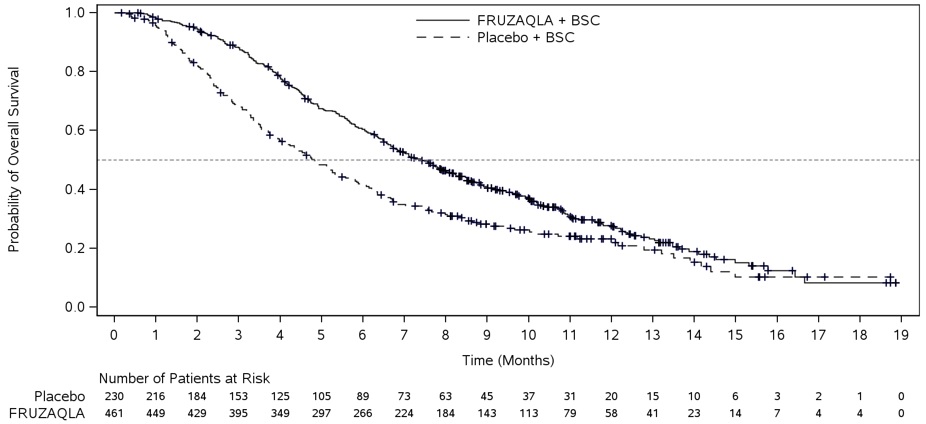
The efficacy of FRUZAQLA was evaluated in FRESCO-2 (NCT04322539), an international, multicenter, randomized, double-blind, placebo-controlled study that enrolled 691 patients with metastatic colorectal cancer who had disease progression during or after prior treatment with fluoropyrimidine-, oxaliplatin-, irinotecan-based chemotherapy, an anti-VEGF biological therapy, if RAS wild type, an anti-EGFR biological therapy, and trifluridine/tipiracil, regorafenib, or both. Patients with an ECOG PS ≥2, left ventricular fraction ≤50%, systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg, urine protein ≥1 g/24h, or untreated brain metastases were ineligible. Randomization was stratified by prior use of trifluridine/tipiracil or regorafenib (trifluridine/tipiracil vs. regorafenib vs. trifluridine/tipiracil and regorafenib), RAS status (wild type vs. mutant), and duration of metastatic disease (≤18 months vs. 18 months).
Patients were randomized (2:1) to receive FRUZAQLA 5 mg orally once daily (N=461) for the first 21 days of each 28-day cycle plus BSC or placebo (N=230) plus BSC. Patients received either FRUZAQLA or placebo until disease progression or unacceptable toxicity. The major efficacy outcome measure was overall survival (OS) and an additional efficacy outcome measure was progression-free survival (PFS) as determined by investigators according to RECIST v1.1.
The study population characteristics were median age of 64 years (range: 25 to 86), with 47% ≥65 years of age; 56% male; 81% White, 9% Asian, 2.9% Black or African American, and 0.7% Native Hawaiian/Pacific Islander; 43% had an ECOG PS of 0 and 57% had an ECOG PS of 1, and 63% had RAS-mutant tumors. Eighteen percent of the patients were enrolled in North America, 72% in Europe, and 10% in Asia Pacific (Japan and Australia) region.
All patients received prior treatment with fluoropyrimidine, oxaliplatin, and irinotecan-based chemotherapy; 96% received prior anti-VEGF therapy, 39% received prior anti-EGFR therapy, 91% received trifluridine/tipiracil, 48% received regorafenib, and 39% received both trifluridine/tipiracil and regorafenib.
The addition of FRUZAQLA to BSC resulted in a statistically significant improvement in OS and PFS compared to placebo plus BSC (see Table 7, Figure 1).
FRESCO Study
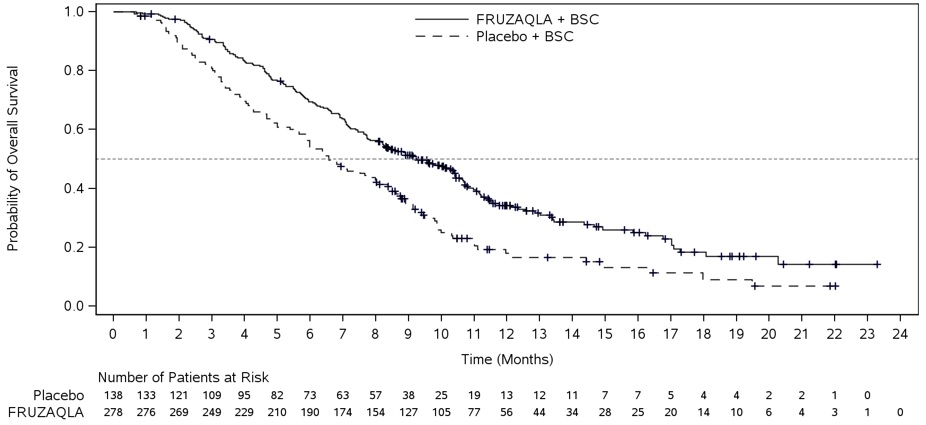
The efficacy of FRUZAQLA was evaluated in FRESCO (NCT02314819), a multicenter, randomized, double-blind, placebo-controlled study conducted in China that enrolled 416 patients with metastatic colorectal cancer who had disease progression during or after prior treatment with fluoropyrimidine-, oxaliplatin, or irinotecan-based chemotherapy. Patients older than 75 years of age, Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥2, left ventricular ejection fraction ≤50%, systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg, urine protein ≥1 g/24h, or brain metastases were ineligible. Randomization was stratified by prior use of VEGF inhibitors (yes vs. no) and K-RAS status (wild type vs. mutant).
Patients were randomized (2:1) to receive FRUZAQLA 5 mg orally once daily (N=278) for the first 21 days of each 28-day cycle plus BSC or placebo (N=138) plus BSC. Patients received either FRUZAQLA or placebo until disease progression or unacceptable toxicity. The major efficacy outcome measure was OS and an additional efficacy outcome measure was PFS as determined by investigators according to RECIST v1.1.
The study population characteristics were median age of 56 years (range: 23 to 75), with 19% ≥65 years of age; 61% male; 100% Asian; 27% had an ECOG PS of 0 and 73% had an ECOG PS of 1 (73%), and 44% had K-RAS mutant tumors.
All patients received prior treatment with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy; 30% of patients received prior anti-VEGF therapy, and 14% received prior anti-EGFR therapy.
The addition of FRUZAQLA to BSC resulted in a statistically significant improvement in OS compared to placebo plus BSC (see Table 7, Figure 2).
Table 7. Efficacy Results from FRESCO-2 and FRESCO Studies:
| FRESCO-2 | FRESCO | |||
|---|---|---|---|---|
| Endpoint | FRUZAQLA + BSC N=461 | Placebo + BSC N=230 | FRUZAQLA + BSC N=278 | Placebo + BSC N=138 |
| OS | ||||
| Number of patients with event (%) | 317 (69%) | 173 (75%) | 188 (68%) | 109 (79%) |
| Median in months (95% CI) | 7.4 (6.7, 8.2) | 4.8 (4.0, 5.8) | 9.3 (8.2, 10.5) | 6.6 (5.9, 8.1) |
| Hazard Ratio* (95% CI) | 0.66 (0.55, 0.80) | 0.65 (0.51, 0.83) | ||
| P-Value† | <0.001 | <0.001 | ||
| PFS | ||||
| Number of patients with event (%) | 392 (85%) | 213 (93%) | 235 (85%) | 125 (91%) |
| Median in months (95% CI) | 3.7 (3.5, 3.8) | 1.8 (1.8, 1.9) | 3.7 (3.7,4.6) | 1.8 (1.8, 1.8) |
| Hazard Ratio* (95% CI) | 0.32 (0.27, 0.39) | 0.26 (0.21, 0.34) | ||
| P-Value†‡ | <0.001 | - | ||
Abbreviations: CI=confidence interval; N=number of patients; OS=overall survival; PFS=progression-free survival
* The Hazard Ratio and its 95% CI were estimated using a stratified Cox proportional hazards model.
† P-Value (2-sided) was calculated using a stratified log-rank test.
‡ P-Value for the PFS analysis in FRESCO was not included due to lack of multiplicity adjustment for this analysis.
Figure 1. Kaplan-Meier Curve for Overall Survival in FRESCO-2:
Figure 2. Kaplan-Meier Curve for Overall Survival in FRESCO:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.