FUSIDIC ACID Sterile viscous eye drops Ref.[6875] Active ingredients: Fusidic acid
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Amdipharm UK Limited, Capital House, 85 King William Street, EC4N 7BL, London, UK
Therapeutic indications
Fusidic acid eye drops are indicated for the topical treatment of bacterial conjunctivitis where the organism is known to be sensitive to the antibiotic.
Posology and method of administration
Posology
For all ages: One Fusidic acid drop to be instilled into the eye twice daily. Treatment should be continued for at least 48 hours after the eye returns to normal.
Method of administration
For ophthalmic use only.
Overdose
The total quantity of fusidic acid in one 5 g tube of Fucithalmic eye drops (50 mg) does not exceed the approved total daily oral dose of fusidic acid contain-ing products. The concentration of the excipients is too low to constitute a safety risk. Therefore, overdose is unlikely to occur.
Shelf life
3 years.
Special precautions for storage
Store below 25°C. Keep the tube tightly closed. The tube should be discarded one month after opening.
Nature and contents of container
Available in 5g tubes.
Special precautions for disposal and other handling
Directions for use for administration
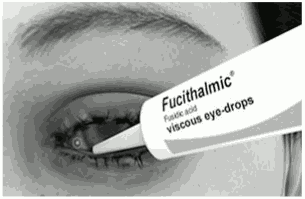
- As with any eye preparation, wash your hands before you administer FUCITHALMIC viscous eye-drops.
- Remove the cap from the tube. To administer FUCITHALMIC viscous eye-drops, stand or sit comfortably and tilt your head backwards. Hold the tube above your eye.
- Gently pull down your lower eyelid and squeeze one drop from the tube into your lower eyelid as shown in the picture. You may find a mirror useful when administering the drops.
- Be careful not to touch the tip of the tube to your eye or other surface, so as to avoid contamination of tube contents.
- FUCITHALMIC viscous eye-drops comes out of the tube as a single viscous drop, which quickly turns to liquid in your eye.
- If the drops are for children, you may put the drops in their eyes when they are lying down or asleep.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.