GEMTESA Film-coated tablet Ref.[50992] Active ingredients: Vibegron
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Vibegron is a selective human beta-3 adrenergic receptor agonist. Activation of the beta-3 adrenergic receptor increases bladder capacity by relaxing the detrusor smooth muscle during bladder filling.
12.2. Pharmacodynamics
Vibegron's exposure-response relationship and the time course of pharmacodynamic response are not fully characterized.
Blood Pressure
In a 4-week, randomized, placebo-controlled, ambulatory blood pressure study in OAB patients (n=200), daily treatment with GEMTESA 75 mg was not associated with clinically significant changes in blood pressure. Subjects enrolled in this study had a mean age of 59 years and 75% were female. Thirty-five percent of subjects had pre-existing hypertension at baseline and 29% of all subjects were taking at least 1 concomitant antihypertensive medication.
Cardiac Electrophysiology
GEMTESA does not prolong the QT interval to any clinically relevant extent at a single dose 5.3 times the approved recommended dose.
12.3. Pharmacokinetics
Mean vibegron Cmax and AUC increased in a greater than dose-proportional manner up to 600 mg (8 times the approved recommended dosage). Steady state concentrations are achieved within 7 days of once daily dosing. The mean accumulation ratio (Rac) was 1.7 for Cmax and 2.4 for AUC0-24hr.
Absorption
Median vibegron Tmax is approximately 1 to 3 hours.
Oral administration of a 75 mg vibegron tablet crushed and mixed with 15 mL of applesauce resulted in no clinically relevant changes in vibegron pharmacokinetics when compared to administration of an intact 75 mg vibegron tablet.
Effect of Food
No clinically significant differences in vibegron pharmacokinetics were observed following administration of a high-fat meal (53% fat, 869 calories [32.1 g protein, 70.2 g carbohydrate, and 51.1 g fat]).
Distribution
The mean apparent volume of distribution is 6304 liters. Human plasma protein binding of vibegron is approximately 50%. The average blood-to-plasma concentration ratio is 0.9.
Elimination
Vibegron has an effective half-life of 30.8 hours across all populations.
Metabolism
Metabolism plays a minor role in the elimination of vibegron. CYP3A4 is the predominant enzyme responsible for in vitro metabolism.
Excretion
Following a radiolabeled dose, approximately 59% of the dose (54% as unchanged) was recovered in feces and 20% (19% as unchanged) in urine.
Specific Populations
No clinically significant differences in the pharmacokinetics of vibegron were observed based on age (18 to 93 years), sex, race/ethnicity (Japanese vs. non-Japanese), mild (eGFR 60 to <90 mL/min/1.73 m²), moderate (eGFR 30 to <60 mL/min/1.73 m²), and severe (eGFR 15 to <30 mL/min/1.73 m²) renal impairment, or moderate (Child-Pugh B) hepatic impairment. The effect of more severe renal impairment (eGFR <15 mL/min/1.73 m²) with or without hemodialysis or severe (Child-Pugh C) hepatic impairment on vibegron pharmacokinetics was not studied.
Drug Interaction Studies
Clinical Studies
Digoxin: Concomitant administration of vibegron increased digoxin Cmax and AUC by 21% and 11%, respectively.
Other Drugs: No clinically significant differences in vibegron pharmacokinetics were observed when used concomitantly with ketoconazole (P-gp and strong CYP3A4 inhibitor), diltiazem (P-gp and moderate CYP3A4 inhibitor), rifampin (strong CYP3A4 inducer), or tolterodine. No clinically significant differences in the pharmacokinetics of the following drugs were observed when used concomitantly with vibegron: tolterodine, tolterodine 5-hydroxy metabolite, metoprolol, combined oral contraceptive (ethinyl estradiol, levonorgestrel), or warfarin.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Vibegron is a CYP3A4 substrate. Vibegron did not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4. Vibegron did not induce CYP1A2, CYP2B6, or CYP3A4.
Transporter Systems: Vibegron is a P-gp substrate. Vibegron did not inhibit P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2, MATE1, or MATE2K at clinically relevant concentrations.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenicity was observed in long-term studies conducted in mice and rats treated with daily oral doses of vibegron for approximately 2 years. In the mouse carcinogenicity study, CD-1 mice were treated with daily oral doses of vibegron up to 90 mg/kg/day in males and up to 150 mg/kg/day in females, corresponding to estimated systemic exposures (AUC) 21- and 55-fold higher, respectively, than in humans treated with the recommended daily dose of GEMTESA. In the rat carcinogenicity study, Sprague Dawley rats were treated with daily oral doses of vibegron up to 30 mg/kg/day in males and up to 180 mg/kg/day in females, corresponding to systemic exposures (AUC) 18- and 117-fold higher, respectively, than in humans treated with the recommended daily dose of GEMTESA.
Mutagenesis
Vibegron was not mutagenic in in vitro microbial reverse mutation assays, showed no evidence of genotoxic activity in an in vitro human peripheral blood lymphocyte chromosomal aberration assay, and did not increase the frequency of micronucleated polychromatic erythrocytes in an in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
In fertility/general reproductive toxicity studies conducted in rats, females were treated with daily oral doses of 0, 30, 100, 300, or 1000 mg/kg/day vibegron and males were treated with daily oral doses of 0, 10, 30, or 300 mg/kg/day vibegron. No effects on fertility were observed in female or male rats at doses up to 300 mg/kg/day, associated with systemic exposure (AUC) at least 274-fold higher than in humans treated with the recommended daily dose of GEMTESA. General toxicity, decreased fecundity, and decreased fertility were observed in female rats at 1000 mg/kg/day, associated with estimated systemic exposure 1867-fold higher than in humans treated with the recommended daily dose of GEMTESA.
14. Clinical Studies
The efficacy of GEMTESA was evaluated in a 12-week, double-blind, randomized, placebo-controlled, and active-controlled trial (Study 3003, NCT03492281) in patients with OAB (urge urinary incontinence, urgency, and urinary frequency). Patients were randomized 5:5:4 to receive either GEMTESA 75 mg, placebo, or active control orally, once daily for 12 weeks. For study entry, patients had to have symptoms of OAB for at least 3 months with an average of 8 or more micturitions per day and at least 1 urge urinary incontinence (UUI) per day, or an average of 8 or more micturitions per day and an average of at least 3 urgency episodes per day. Urge urinary incontinence was defined as leakage of urine of any amount because the patient felt an urge or need to urinate immediately. The study population included OAB medication-naïve patients as well as patients who had received prior therapy with OAB medications.
The co-primary endpoints were change from baseline in average daily number of micturitions and average daily number of UUI episodes at week 12. Additional endpoints included change from baseline in average daily number of "need to urinate immediately" (urgency) episodes and average volume voided per micturition.
A total of 1,515 patients received at least one daily dose of placebo (n=540), GEMTESA 75 mg (n=545), or an active control treatment (n=430). The majority of patients were Caucasian (78%) and female (85%) with a mean age of 60 (range 18 to 93) years.
Table 2 shows changes from baseline at week 12 for average daily number of micturitions, average daily number of UUI episodes, average daily number of "need to urinate immediately" (urgency) episodes, and average volume voided per micturition.
Table 2. Mean Baseline and Change from Baseline at Week 12 for Micturition Frequency, Urge Urinary Incontinence Episodes, "Need to Urinate Immediately" (Urgency) Episodes, and Volume Voided per Micturition:
| Parameter | GEMTESA 75 mg | Placebo |
|---|---|---|
| Average Daily Number of Micturitions | ||
| Baseline mean (n) | 11.3 (526) | 11.8 (520) |
| Change from Baseline* (n) | -1.8 (492) | -1.3 (475) |
| Difference from Placebo | -0.5 | |
| 95% Confidence Interval | -0.8, -0.2 | |
| p-value | <0.001 | |
| Average Daily Number of UUI Episodes | ||
| Baseline mean (n) | 3.4 (403) | 3.5 (405) |
| Change from Baseline* (n) | -2.0 (383) | -1.4 (372) |
| Difference from Placebo | -0.6 | |
| 95% Confidence Interval | -0.9, -0.3 | |
| p-value | <0.0001 | |
| Average Daily Number of "Need to Urinate Immediately" (Urgency) Episodes | ||
| Baseline mean (n) | 8.1 (526) | 8.1 (520) |
| Change from Baseline* (n) | -2.7 (492) | -2.0 (475) |
| Difference from Placebo | -0.7 | |
| 95% Confidence Interval | -1.1, -0.2 | |
| p-value | 0.002 | |
| Average Volume Voided (mL) per Micturition | ||
| Baseline mean (n) | 155 (524) | 148 (514) |
| Change from Baseline* (n) | 23 (490) | 2 (478) |
| Difference from Placebo | 21 | |
| 95% Confidence Interval | 14, 28 | |
| p-value | <0.0001 | |
* Least squares mean adjusted for treatment, baseline, sex, geographical region, study visit, and study visit by treatment interaction term.
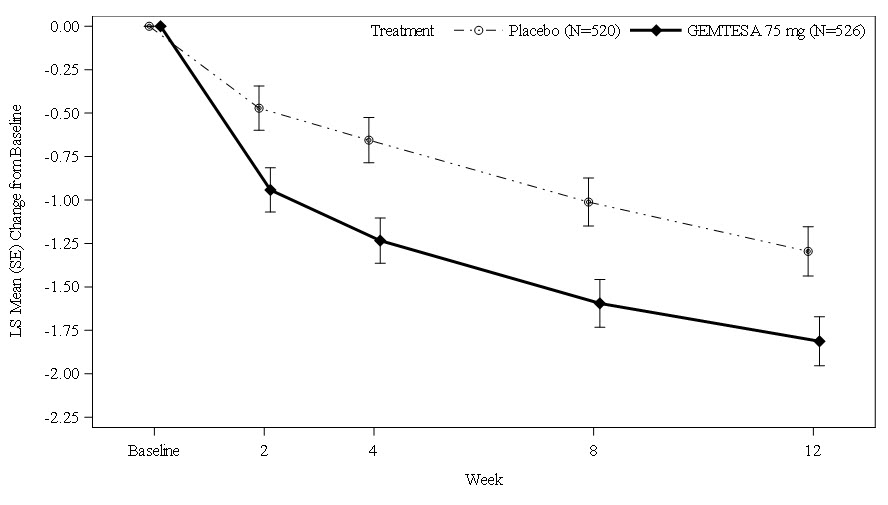
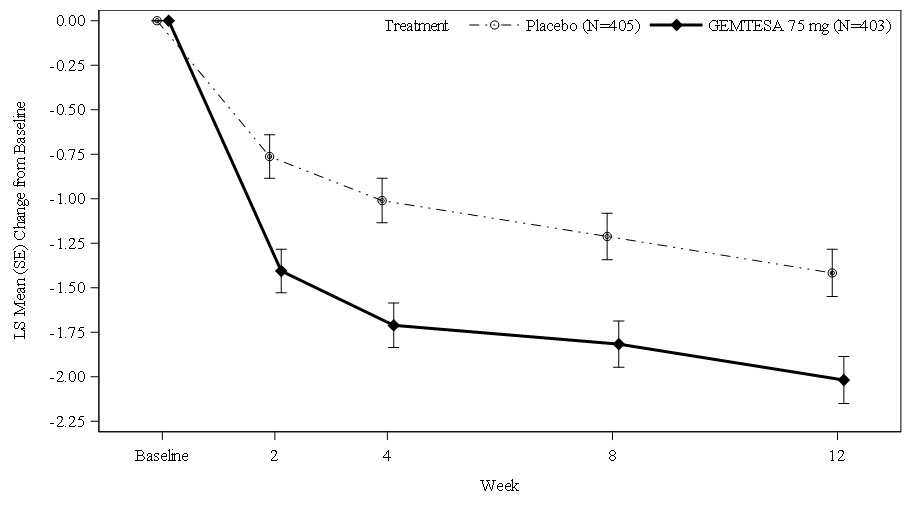
Figures 1 and 2 show the mean change from baseline over time in average daily number of micturitions and mean change from baseline over time in average daily number of UUI episodes, respectively.
Figure 1. Mean (SE) Change from Baseline in the Average Daily Number of Micturitions:
Figure 2. Mean (SE) Change from Baseline in the Average Daily Number of UUI Episodes in Patients with At Least 1 Average Daily UUI Episode at Baseline:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.