GHEMAXAN Solution for injection Ref.[49862] Active ingredients: Enoxaparin
Source: Health Products Regulatory Authority (IE) Revision Year: 2022 Publisher: Chemi S.p.A., Via dei Lavoratori, 54, Cinisello Balsamo (MI), 20092 Italy
4.1. Therapeutic indications
Ghemaxan is indicated in adults for:
- Prophylaxis of venous thromboembolic disease in moderate and high-risk surgical patients, in particular those undergoing orthopaedic or general surgery including cancer surgery.
- Prophylaxis of venous thromboembolic disease in medical patients with an acute illness (such as acute heart failure, respiratory insufficiency, severe infections or rheumatic diseases) and reduced mobility at increased risk of venous thromboembolism.
- Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), excluding PE likely to require thrombolytic therapy or surgery.
- Prevention of thrombus formation in the extracorporeal circulation during haemodialysis.
*Acute coronary syndrome:
- Treatment of unstable angina and non ST-segment elevation myocardial infarction (NSTEMI), in combination with oral acetylsalicylic acid.
- Treatment of acute ST-segment elevation myocardial infarction (STEMI) including patients to be managed medically or with subsequent percutaneous coronary intervention (PCI).
4.2. Posology and method of administration
Posology
Prophylaxis of venous thromboembolic disease in moderate and high-risk surgical patients
- Individual thromboembolic risk for patients can be estimated using a validated risk stratification model
In patients at moderate risk of thromboembolism, the recommended dose of enoxaparin sodium is 2,000 IU (20 mg) once daily by subcutaneous (SC) injection. Preoperative initiation (2 hours before surgery) of enoxaparin sodium 2,000 IU (20 mg) was proven effective and safe in moderate‑risk surgery.
In moderate‑risk patients, enoxaparin sodium treatment should be maintained for a minimal period of 7‑10 days whatever the recovery status (e.g. mobility). Prophylaxis should be continued until the patient no longer has significantly reduced mobility.
- In patients at high risk of thromboembolism, the recommended dose of enoxaparin sodium is 4,000 IU (40 mg) once daily given by SC preferably started 12 hours before surgery.
If there is a need for earlier than 12 hours enoxaparin sodium preoperative prophylactic initiation (e.g. high-risk patient waiting for a deferred orthopaedic surgery), the last injection should be administered no later than 12 hours prior to surgery and resumed 12 hours after surgery.
- For patients who undergo major orthopaedic surgery, extended thromboprophylaxis up to 5 weeks is recommended.
- For patients with a high venous thromboembolism (VTE) risk who undergo abdominal or pelvic surgery for cancer, extended thromboprophylaxis up to 4 weeks is recommended.
Prophylaxis of venous thromboembolism in medical patients
The recommended dose of enoxaparin sodium is 4,000 IU (40 mg) once daily by SC injection. Treatment with enoxaparin sodium is prescribed for at least 6 to 14 days whatever the recovery status (e.g. mobility). The benefit is not established for treatment longer than 14 days.
Treatment of DVT and PE
Enoxaparin sodium can be administered SC either as a once-daily injection of 150 IU/kg (1.5 mg/kg) or as a twice-daily injection of 100 IU/kg (1 mg/kg).
The regimen should be selected by the physician based on an individual assessment including evaluation of the thromboembolic risk and of the risk of bleeding. The dose regimen of 150 IU/kg (1.5 mg/kg) administered once daily should be used in uncomplicated patients with low risk of VTE recurrence. The dose regimen of 100 IU/kg (1 mg/kg) administered twice daily should be used in all other patients such as those with obesity, with symptomatic PE, cancer, recurrent VTE or proximal (vena iliaca) thrombosis.
Enoxaparin sodium treatment is prescribed for an average period of 10 days. Oral anticoagulant therapy should be initiated when appropriate (see “Switch between enoxaparin sodium and oral anticoagulants” at the end of section 4.2).
Prevention of thrombus formation during haemodialysis
The recommended dose is 100 IU/kg (1 mg/kg) of enoxaparin sodium. For patients with a high risk of haemorrhage, the dose should be reduced to 50 IU/kg (0.5 mg/kg) for double vascular access or 75 IU/kg (0.75 mg/kg) for single vascular access.
During haemodialysis, enoxaparin sodium should be introduced into the arterial line of the circuit at the beginning of the dialysis session. The effect of this dose is usually sufficient for a 4-hour session; however, if fibrin rings are found, for example after a longer than normal session, a further dose of 50 IU to 100 IU/kg (0.5 to 1 mg/kg) may be given.
No data are available in patients using enoxaparin sodium for prophylaxis or treatment and during haemodialysis sessions.
Acute coronary syndrome: treatment of unstable angina and NSTEMI and treatment of acute STEMI
- For treatment of unstable angina and NSTEMI, the recommended dose of enoxaparin sodium is 100 IU/kg (1 mg/kg) every 12 hours by SC injection, administered in combination with antiplatelet therapy. Treatment should be maintained for a minimum of 2 days and continued until clinical stabilisation. The usual duration of treatment is 2 to 8 days. Acetylsalicylic acid is recommended for all patients without contraindications at an initial oral loading dose of 150–300 mg (in acetylsalicylic acid-naïve patients) and a maintenance dose of 75‑325 mg/day long-term regardless of treatment strategy.
- For treatment of acute STEMI, the recommended dose of enoxaparin sodium is a single intravenous (IV) bolus of 3,000 IU (30 mg) plus a 100 IU/kg (1 mg/kg) SC dose followed by 100 IU/kg (1 mg/kg) administered SC every 12 hours (maximum 10,000 IU (100 mg) for each of the first two SC doses). Appropriate antiplatelet therapy such as oral acetylsalicylic acid (75 mg to 325 mg once daily) should be administered concomitantly unless contraindicated. The recommended duration of treatment is 8 days or until hospital discharge, whichever comes first. When administered in conjunction with a thrombolytic (fibrin-specific or non-fibrin-specific), enoxaparin sodium should be given between 15 minutes before and 30 minutes after the start of fibrinolytic therapy.
- For dosage in patients ≥75 years of age, see paragraph “Elderly”.
- For patients managed with PCI, if the last dose of enoxaparin sodium SC was given less than 8 hours before balloon inflation, no additional dosing is needed. If the last SC administration was given more than 8 hours before balloon inflation, an IV bolus of 30 IU/kg (0.3 mg/kg) enoxaparin sodium should be administered.
Paediatric population
The safety and efficacy of enoxaparin sodium in the paediatric population have not been established.
Elderly
For all indications except STEMI, no dose reduction is necessary in elderly patients, unless kidney function is impaired (see below “Renal impairment” and section 4.4).
For treatment of acute STEMI in elderly patients ≥75 years of age, an initial IV bolus must not be used. Initiate dosing with 75 IU/kg (0.75 mg/kg) SC every 12 hours (maximum 7,500 IU (75 mg) for each of the first two SC doses only, followed by 75 IU/kg (0.75 mg/kg) SC dosing for the remaining doses). For dosage in elderly patients with impaired kidney function, see below “Renal impairment” and section 4.4.
Hepatic impairment
Limited data are available in patients with hepatic impairment (see sections 5.1 and 5.2) and caution should be exercised in these patients (see section 4.4).
Renal impairment (see sections 4.4 and 5.2)
h4, Severe renal impairment
Enoxaparin sodium is not recommended for patients with end-stage renal disease (creatinine clearance <15 mL/min) due to lack of data in this population outside the prevention of thrombus formation in extracorporeal circulation during haemodialysis.
Dosage table for patients with severe renal impairment (creatinine clearance 15‑30 mL/min):
| Indication | Dosing regimen |
|---|---|
| Prophylaxis of venous thromboembolic disease | 2,000IU (20mg) SC once daily |
| Treatment of DVT and PE | 100IU/kg (1mg/kg) body weight SC once daily |
| Treatment of unstable angina and NSTEMI | 100IU/kg (1mg/kg) body weight SC once daily |
| Treatment of acute STEMI (patients under 75 years) Treatment of acute STEMI (patients over 75 years) | 1 × 3,000IU (30mg) IV bolus plus 100IU/kg (1mg/kg) body weight SC and then 100IU/kg (1mg/kg) body weight SC every 24hours No IV initial bolus, 100IU/kg (1mg/kg) body weight SC and then 100IU/kg (1mg/kg) body weight SC every 24hours |
The recommended dosage adjustments do not apply to the haemodialysis indication.
Moderate and mild renal impairment
Although no dosage adjustment is recommended in patients with moderate (creatinine clearance 30‑50 mL/min) and mild (creatinine clearance 50‑80 mL/min) renal impairment, careful clinical monitoring is advised.
Method of administration
Ghemaxan should not be administered by the intramuscular route.
For the prophylaxis of venous thromboembolic disease following surgery, treatment of DVT and PE, treatment of unstable angina and NSTEMI, enoxaparin sodium should be administered by SC injection.
- For acute STEMI, treatment is to be initiated with a single IV bolus injection immediately followed by a SC injection.
- For the prevention of thrombus formation in the extracorporeal circulation during haemodialysis, it is administered through the arterial line of a dialysis circuit.
The pre-filled disposable syringe is ready for immediate use.
SC injection technique:
Injection should be made preferably when the patient is lying down. Enoxaparin sodium is administered by deep SC injection.
Do not expel the air bubble from the syringe before the injection to avoid the loss of medicinal product when using pre-filled syringes. When the quantity of medicinal product to be injected needs adjusting based on the patient’s body weight, use the graduated pre-filled syringes to reach the required volume by discarding the excess before injection. Please be aware that, in some cases, it is not possible to achieve an exact dose due to the graduations on the syringe and, in such cases, the volume shall be rounded up to the nearest graduation.
Administration should be alternated between the left and right anterolateral or posterolateral abdominal wall. The whole length of the needle should be introduced vertically into a skin fold held between the thumb and index finger. The skin fold should not be released until the injection is complete. Do not rub the injection site after administration.
Note that for the pre-filled syringes equipped with a needle shield guard, the safety system is triggered at the end of the injection (see instructions in section 6.6).
In case of self-administration, patients should be advised to follow instructions provided in the patient information leaflet included in the pack of this medicine.
IV (bolus) injection (for acute STEMI indication only):
For acute STEMI, treatment is to be initiated with a single IV bolus injection immediately followed by a SC injection. For IV injection, either the multidose vial or pre-filled syringe can be used. Enoxaparin sodium should be administered through an IV line. It should not be mixed or co-administered with other medicinal products. To avoid the possible mixture of enoxaparin sodium with all other medicinal products, the IV access chosen should be flushed with a sufficient amount of saline or dextrose solution prior to and following the IV bolus administration of enoxaparin sodium to clear the port of medicinal product. Enoxaparin sodium may be safely administered with normal saline solution (0.9%) or 5% dextrose in water.
- Initial 3,000 IU (30 mg) bolus
For the initial 3,000 IU (30 mg) bolus, using an enoxaparin sodium graduated pre-filled syringe, expel the excess volume to retain only 3,000 IU (30 mg) in the syringe. The 3,000 IU (30 mg) dose can then be directly injected into an injection site in the intravenous line.
- Additional bolus for PCI when last SC administration was given more than 8 hours before balloon inflation
For patients being managed with PCI, an additional IV bolus of 30 IU/kg (0.3 mg/kg) is to be administered if last SC administration was given more than 8 hours before balloon inflation.
In order to assure the accuracy of the small volume to be injected, it is recommended to dilute the medicinal product to 300 IU/mL (3 mg/mL).
To obtain a 300 IU/mL (3 mg/mL) solution, using a 6,000 IU (60 mg) enoxaparin sodium pre-filled syringe, it is recommended to use a 50 mL infusion bag (i.e. using either normal saline solution (0.9%) or 5% dextrose in water) as follows: Withdraw 30 mL from the infusion bag with a syringe and discard the liquid. Inject the complete contents of the 6,000 IU (60 mg) enoxaparin sodium pre-filled syringe into the 20 mL remaining in the bag. Gently mix the contents of the bag. Withdraw the required volume of diluted solution with a syringe for administration into the IV line.
After dilution is completed, the volume to be injected can be calculated using the following formula [Volume of diluted solution (mL) = Patient weight (kg) × 0.1] or using the table below. It is recommended that the dilution be prepared immediately before use.
Volume to be injected through IV line after dilution is completed at a concentration of 300 IU (3 mg)/mL:
| Weight | Required dose 30 IU/kg (0.3 mg/kg) | Volume to inject when diluted to a final concentration of 300 IU (3 mg)/mL | |
|---|---|---|---|
| [Kg] | IU | [mg] | [mL] |
| 45 | 1350 | 13.5 | 4.5 |
| 50 | 1500 | 15 | 5 |
| 55 | 1650 | 16.5 | 5.5 |
| 60 | 1800 | 18 | 6 |
| 65 | 1950 | 19.5 | 6.5 |
| 70 | 2100 | 21 | 7 |
| 75 | 2250 | 22.5 | 7.5 |
| 80 | 2400 | 24 | 8 |
| 85 | 2550 | 25.5 | 8.5 |
| 90 | 2700 | 27 | 9 |
| 95 | 2850 | 28.5 | 9.5 |
| 100 | 3000 | 30 | 10 |
| 105 | 3150 | 31.5 | 10.5 |
| 110 | 3300 | 33 | 11 |
| 115 | 3450 | 34.5 | 11.5 |
| 120 | 3600 | 36 | 12 |
| 125 | 3750 | 37.5 | 12.5 |
| 130 | 3900 | 39 | 13 |
| 135 | 4050 | 40.5 | 13.5 |
| 140 | 4200 | 42 | 14 |
| 145 | 4350 | 43.5 | 14.5 |
| 150 | 4500 | 45 | 15 |
- Arterial line injection:
It is administered through the arterial line of a dialysis circuit for the prevention of thrombus formation in the extracorporeal circulation during haemodialysis.
Switch between enoxaparin sodium and oral anticoagulants
Switch between enoxaparin sodium and vitamin K antagonists (VKA)
Clinical monitoring and laboratory tests [prothrombin time expressed as the International Normalised Ratio (INR)] must be intensified to monitor the effect of VKA.
As there is an interval before the VKA reaches its maximum effect, enoxaparin sodium therapy should be continued at a constant dose for as long as necessary in order to maintain the INR within the desired therapeutic range for the indication in two successive tests.
For patients currently receiving a VKA, the VKA should be discontinued and the first dose of enoxaparin sodium should be given when the INR has dropped below the therapeutic range.
Switch between enoxaparin sodium and direct oral anticoagulants (DOAC)
For patients currently receiving enoxaparin sodium, discontinue enoxaparin sodium and start the DOAC 0 to 2 hours before the time that the next scheduled administration of enoxaparin sodium would be due as per DOAC label.
For patients currently receiving a DOAC, the first dose of enoxaparin sodium should be given at the time the next DOAC dose would be taken.
Administration in spinal/epidural anaesthesia or lumbar puncture
Should the physician decide to administer anticoagulation in the context of epidural or spinal anaesthesia/analgesia or lumbar puncture, careful neurological monitoring is recommended due to the risk of neuraxial haematomas (see section 4.4).
At doses used for prophylaxis
A puncture-free interval of at least 12 hours shall be kept between the last injection of enoxaparin sodium at prophylactic doses and the needle or catheter placement.
For continuous techniques, a similar delay of at least 12 hours should be observed before removing the catheter.
For patients with creatinine clearance of 15‑30 mL/min, consider doubling the timing of puncture/catheter placement or removal to at least 24 hours.
The 2-hour preoperative initiation of enoxaparin sodium 2,000 IU (20 mg) is not compatible with neuraxial anaesthesia.
At doses used for treatment
A puncture-free interval of at least 24 hours shall be kept between the last injection of enoxaparin sodium at curative doses and the needle or catheter placement (see also section 4.3). For continuous techniques, a similar delay of 24 hours should be observed before removing the catheter. For patients with creatinine clearance of 15‑30 mL/min, consider doubling the timing of puncture/catheter placement or removal to at least 48 hours. Patients receiving the twice-daily doses (i.e. 75 IU/kg (0.75 mg/kg) twice daily or 100 IU/kg (1 mg/kg) twice daily) should omit the second enoxaparin sodium dose to allow a sufficient delay before catheter placement or removal.
Anti-Xa levels are still detectable at these time points and these delays are not a guarantee that neuraxial haematoma will be avoided.
Likewise, consider not using enoxaparin sodium until at least 4 hours after the spinal/epidural puncture or after the catheter has been removed. The delay must be based on a benefit-risk assessment, considering both the risk for thrombosis and the risk for bleeding in the context of the procedure and patient risk factors.
4.9. Overdose
Signs and symptoms
Accidental overdose with enoxaparin sodium after IV, extracorporeal or SC administration may lead to haemorrhagic complications. Following oral administration of even large doses, it is unlikely that enoxaparin sodium will be absorbed.
Management
The anticoagulant effects can be largely neutralised by the slow IV injection of protamine. The dose of protamine depends on the dose of enoxaparin sodium injected; 1 mg protamine neutralises the anticoagulant effect of 100 IU (1 mg) of enoxaparin sodium, if enoxaparin sodium was administered in the previous 8 hours. An infusion of 0.5 mg protamine per 100 IU (1 mg) of enoxaparin sodium may be administered if enoxaparin sodium was administered more than 8 hours previous to the protamine administration, or if it has been determined that a second dose of protamine is required. After 12 hours of the enoxaparin sodium injection, protamine administration may not be required. However, even with high doses of protamine, the anti-Xa activity of enoxaparin sodium is never completely neutralised (maximum about 60%) (see the prescribing information for protamine salts).
6.3. Shelf life
3 years.
6.4. Special precautions for storage
Do not freeze.
This medicinal product is for single use only. Discard any unused product.
6.5. Nature and contents of container
Solution for injection in a 0.5 mL or 1 mL Type I glass pre-filled syringe with staked needle and needle guard (synthetic polyisoprene rubber) closed with elastomeric plunger stopper (chlorobutyl rubber) and plunger rod. The solution for injection is available in two different presentations:
1. The syringe is equipped with a needle guard
Ghemaxan 2,000 IU (20 mg)/0.2 mL solution for injection in pre-filled syringes: packs of 2, 6 or 10 pre-filled syringes and multipacks containing 12 (2 packs of 6), 20 (2 packs of 10), 24 (4 packs of 6), 30 (3 packs of 10), 50 (5 packs of 10) and 90 (9 packs of 10) pre-filled syringes.
2. The syringe is not equipped with a needle guard
Ghemaxan 2,000 IU (20 mg)/0.2 mL solution for injection in pre-filled syringes: packs of 2 and 10 pre-filled syringes.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
The pre-filled syringe is ready for immediate use (see section 4.2).
For intravenous injection, the product can be diluted in normal saline (0.9%) or 5% dextrose in water.
The solution should be inspected visually prior to use. It must not be used if there is any change in the appearance of the solution.
The Ghemaxan pre-filled syringes are for single dose use only; discard any unused medicinal product.
Pre-filled syringes are supplied with or without a needle guard. The instructions for use are presented in the package leaflet.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
INSTRUCTIONS FOR USE: PRE-FILLED SYRINGE
How to give yourself an injection of Ghemaxan
If you are able to give Ghemaxan to yourself, your doctor or nurse will show you how to do this. Do not try to inject yourself if you have not been trained how to do so. If you are not sure what to do, talk to your doctor or nurse immediately.
Before injecting yourself with Ghemaxan
- Check the expiry date on the medicine. Do not use if the date has passed.
- Check the syringe is not damaged and the medicine in it is a clear solution. If not, use another syringe.
- Do not use this medicine if you notice any change in the appearance of the product.
- Make sure you know how much you are going to inject.
- Check your abdomen to see if the last injection caused any redness, change in skin colour, swelling, oozing or is still painful, if so, talk to your doctor or nurse.
- Decide where you are going to inject the medicine. Change the place where you inject each time from the right to the left side of your stomach. Ghemaxan should be injected just under the skin on your stomach, but not too near the belly button or any scar tissue (at least 5 cm away from these).
The pre-filled syringe is intended for single, one-time use only and are available in the following presentations:
- with a needle guard
- without a needle guard
Instructions on injecting yourself with Ghemaxan
You should be lying down and Ghemaxan administered by deep SC injection. Administration should be alternated between the left and right anterolateral and left and right posterolateral abdominal wall. The whole length of the needle should be introduced into a skin fold held between the thumb and forefinger; the skin fold should be held throughout the injection. To minimise bruising, do not rub the injection site after completion of the injection.
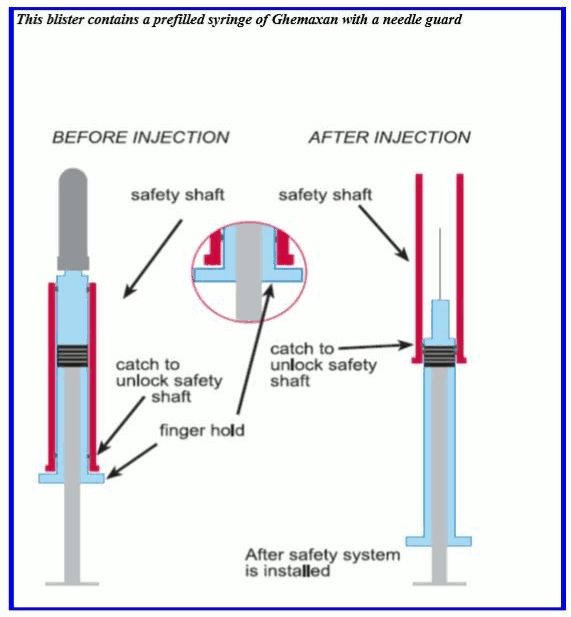
Ghemaxan pre-filled syringes and graduated pre-filled syringes are for single, one-time use only. The syringes may be equipped with a needle guard; instructions for using syringes with this system are given below.
The safety shaft is provided with a catch to unlock and lock the system.
Remove the pre-filled syringe from the blister packaging by peeling at the arrow as directed on the blister. Do not remove by pulling on the plunger, as this may damage the syringe.
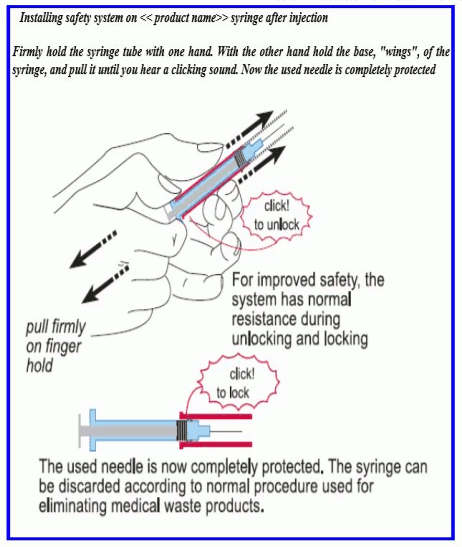
INSTRUCTIONS FOR USE: SAFETY DEVICE
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.