GLUCOPHAGE Tablet / Extended-release tablet Ref.[10564] Active ingredients: Metformin
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
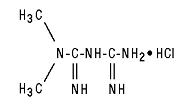
GLUCOPHAGE/GLUCOPHAGE XR contain the antihyperglycemic agent metformin, which is a biguanide, in the form of monohydrochloride. The chemical name of metformin hydrochloride is N,N-dimethylimidodicarbonimidic diamide hydrochloride. The structural formula is as shown below:
Metformin hydrochloride is a white to off-white crystalline compound with a molecular formula of C4H11N5 • HCl and a molecular weight of 165.63. It is freely soluble in water and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68.
GLUCOPHAGE tablets contain 500 mg, 850 mg, or 1000 mg of metformin hydrochloride, which is equivalent to 389.93 mg, 662.88 mg, 779.86 mg metformin base, respectively. Each tablet contains the inactive ingredients povidone and magnesium stearate. In addition, the coating for the 500 mg and 850 mg tablets contains hypromellose and the coating for the 1000 mg tablet contains hypromellose and polyethylene glycol.
GLUCOPHAGE XR contains 500 mg or 750 mg of metformin hydrochloride, which is equivalent to 389.93 mg, 584.90 mg metformin base, respectively.
GLUCOPHAGE XR 500 mg tablets contain the inactive ingredients hypromellose, microcrystalline cellulose, sodium carboxymethyl cellulose, and magnesium stearate.
GLUCOPHAGE XR 750 mg tablets contain the inactive ingredients hypromellose, sodium carboxymethyl cellulose, magnesium stearate and iron oxide pigment red.
| Dosage Forms and Strengths |
|---|
|
GLUCOPHAGE is available as:
GLUCOPHAGE XR is available as:
|
| How Supplied | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 12. GLUCOPHAGE/GLUCOPHAGE XR Available Strengths, Units, and Appearance:
Distributed by: Bristol-Myers Squibb Company, Princeton, NJ 08543, USA |
||||||||||||||||||||||||||||
Drugs
| Drug | Countries | |
|---|---|---|
| GLUCOPHAGE | Albania, Austria, Canada, Cyprus, Germany, Estonia, Finland, France, Hong Kong, Croatia, Ireland, Italy, Malta, Nigeria, Poland, Romania, Singapore, Tunisia, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.