GLUCOTROL XL Extended-release tablet Ref.[10567] Active ingredients: Glipizide
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
GLUCOTROL XL (glipizide) is an oral sulfonylurea.
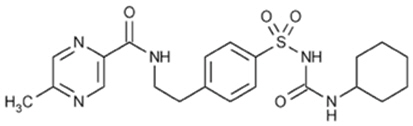
The Chemical Abstracts name of glipizide is 1-cyclohexyl-3-[[p-[2-(5-methylpyrazinecarboxamido)ethyl] phenyl]sulfonyl]urea. The molecular formula is C21H27N5O4S; the molecular weight is 445.55; the structural formula is shown below:
Glipizide is a whitish, odorless powder with a pKa of 5.9. It is insoluble in water and alcohols, but soluble in 0.1 N NaOH; it is freely soluble in dimethylformamide.
Inert ingredients in the 2.5 mg, 5 mg and 10 mg formulations are: polyethylene oxide, hypromellose, magnesium stearate, sodium chloride, red ferric oxide, cellulose acetate, polyethylene glycol, Opadry blue (OY-LS-20921)(2.5 mg tablets), Opadry white (YS-2-7063)(5 mg and 10 mg tablet) and Opacode Black Ink (S-1-17823).
System Components and Performance
GLUCOTROL XL Extended Release Tablet is similar in appearance to a conventional tablet. It consists, however, of an osmotically active drug core surrounded by a semipermeable membrane. The core itself is divided into two layers: an "active" layer containing the drug, and a "push" layer containing pharmacologically inert (but osmotically active) components. The membrane surrounding the tablet is permeable to water but not to drug or osmotic excipients. As water from the gastrointestinal tract enters the tablet, pressure increases in the osmotic layer and "pushes" against the drug layer, resulting in the release of drug through a small, laser-drilled orifice in the membrane on the drug side of the tablet.
The function of the GLUCOTROL XL Extended Release Tablet depends upon the existence of an osmotic gradient between the contents of the bi-layer core and fluid in the GI tract. The biologically inert components of the tablet remain intact during GI transit and are eliminated in the feces as an insoluble shell.
| Dosage Forms and Strengths |
|---|
|
GLUCOTROL XL (glipizide) Extended Release tablets: 2.5 mg, blue and imprinted with "GLUCOTROL XL 2.5" or "GXL 2.5" on one side. 5 mg, white and imprinted with "GLUCOTROL XL 5" or "GXL 5" on one side. 10 mg, white and imprinted with "GLUCOTROL XL 10" or "GXL 10" on one side. |
| How Supplied | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
GLUCOTROL XL (glipizide) Extended Release Tablets are supplied as 2.5 mg, 5 mg, and 10 mg round, biconvex tablets and imprinted with black ink as follows: Table 2. GLUCOTROL XL Tablet Presentations:
|
Drugs
| Drug | Countries | |
|---|---|---|
| GLUCOTROL | Estonia, Hong Kong, Lithuania, Romania, Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.