HALOG Ointment Ref.[10101] Active ingredients: Halcinonide
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
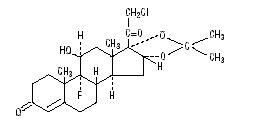
The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. The steroids in this class include halcinonide. Halcinonide is designated chemically as 21-Chloro-9-fluoro-11β, 16α, 17-trihydroxypregn-4-ene-3,20-dione cyclic 16,17-acetal with acetone.
Graphic formula:
C24H32ClFO5, MW 454.96, CAS-3093-35-4
Each gram of 0.1% HALOG OINTMENT (Halcinonide Ointment, USP) contains 1 mg halcinonide in Plastibase (Plasticized Hydrocarbon Gel), a mineral oil and polyethylene gel base, polyethylene glycol 300, polyethylene glycol 400, polyethylene glycol 1450, and polyethylene glycol 6000 distearate with butylated hydroxytoluene as an antioxidant.
| How Supplied |
|---|
|
HALOG OINTMENT (Halcinonide Ointment, USP) 0.1% is translucent white to off-white, smooth, soft homogeneous ointment type material, essentially free of foreign matter and is supplied as:
Manufactured by: DPT Laboratories Inc., San Antonio, TX 78215 Distributed by: Sun Pharmaceutical Industries, Inc., Cranbury, NJ 08512 |
Drugs
| Drug | Countries | |
|---|---|---|
| HALOG | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.