HECTOROL Capsule / Solution for injection Ref.[10031] Active ingredients: Doxercalciferol
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
HECTOROL contains doxercalciferol, which is a synthetic vitamin D2 analog. Doxercalciferol undergoes metabolic activation in vivo to form 1α,25-dihydroxyvitamin D2 (1α,25-(OH)2D2), a naturally occurring, biologically active form of vitamin D2.
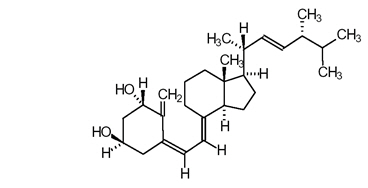
Doxercalciferol is a colorless crystalline compound with a calculated molecular weight of 412.66 and a molecular formula of C28H44O2. It is soluble in oils and organic solvents, but is relatively insoluble in water. Chemically, doxercalciferol is (1α,3β,5Z,7E,22E)-9,10-secoergosta-5,7,10(19),22-tetraene-1,3-diol. The structural formula is:
Capsules
HECTOROL capsules are soft gelatin capsules containing 0.5 mcg, 1 mcg, or 2.5 mcg doxercalciferol for oral use. Each capsule also contains butylated hydroxyanisole (BHA), ethanol, and fractionated triglyceride of coconut oil. The capsule shells contain gelatin, glycerin, and titanium dioxide. In addition, the 0.5 mcg capsule shells contain yellow iron oxide and FD&C Red No. 40, the 1 mcg capsule shells contain FD&C Yellow No. 6, and the 2.5 mcg capsule shells contain yellow iron oxide.
Injection
HECTOROL injection 1 mL single-dose vials contain 2 mcg/mL of doxercalciferol. HECTOROL injection 2 mL single-dose vials contain 4 mcg/2 mL (2 mcg/mL) of doxercalciferol. Each milliliter (mL) of solution contains 2 mcg doxercalciferol and the following inactive ingredients: butylated hydroxytoluene (0.02 mg); disodium edetate (1.1 mg); ethanol, 100% (0.05 mL); polysorbate 20 (10 mg); sodium chloride (1.5 mg); sodium phosphate dibasic, heptahydrate (14.4 mg); and sodium phosphate monobasic, monohydrate (1.8 mg).
HECTOROL injection 2 mL multiple-dose vials contain 4 mcg/2 mL (2 mcg/mL) of doxercalciferol. Each milliliter (mL) of solution contains 2 mcg doxercalciferol and the following inactive ingredients: butylated hydroxytoluene (0.02 mg); disodium edetate (1.1 mg); ethanol, 100% (0.075 mL); polysorbate 20 (10 mg); sodium chloride (1.5 mg); sodium phosphate dibasic, heptahydrate (14.4 mg); and sodium phosphate monobasic, monohydrate (1.8 mg).
| Dosage Forms and Strengths |
|---|
|
Capsules: soft gelatin, oval capsules with imprinted “g” available as follows:
Injection: clear and colorless solution available as follows:
|
| How Supplied | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
HECTOROL capsules are oval, soft gelatin capsules supplied as follows.
HECTOROL injection is a clear, colorless solution supplied in 2 mL amber glass vials as follows.
|
Drugs
| Drug | Countries | |
|---|---|---|
| HECTOROL | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.