HEMLIBRA Solution for injection Ref.[8987] Active ingredients: Emicizumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Roche Registration GmbH, Emil-Barell-Strasse 1, 79639, Grenzach-Wyhlen, Germany
Pharmacodynamic properties
Pharmacotherapeutic group: antihemorrhagics, other systemic hemostatics
ATC code: B02BX06
Mechanism of action
Emicizumab is a humanized monoclonal modified immunoglobulin G4 (IgG4) antibody with a bispecific antibody structure.
Emicizumab bridges activated factor IX and factor X to restore the function of missing FVIIIa that is needed for effective haemostasis.
Emicizumab has no structural relationship or sequence homology to FVIII and, as such, does not induce or enhance the development of direct inhibitors to FVIII.
Pharmacodynamics
Prophylactic therapy with Hemlibra shortens the aPTT and increases the reported FVIII activity (using a chromogenic assay with human coagulation factors). These two pharmacodynamic markers do not reflect the true haemostatic effect of emicizumab in vivo (aPTT is overly shortened and reported FVIII activity may be overestimated) but provide a relative indication of the pro-coagulant effect of emicizumab.
Clinical efficacy and safety
The efficacy of Hemlibra for routine prophylaxis in patients with haemophilia A was evaluated in five clinical studies (three adult and adolescent studies in patients with haemophilia A with or without FVIII inhibitors [HAVEN 1, HAVEN 3, and HAVEN 4], a paediatric study in patients with haemophilia A with FVIII inhibitors [HAVEN 2] and an all-age group study in patients with mild or moderate haemophilia A without FVIII inhibitors [HAVEN 6]).
Clinical studies in adult and adolescent patients with haemophilia A with or without FVIII inhibitors
Patients (aged ≥12 years old and >40 kg) with haemophilia A without FVIII inhibitors (Study BH30071 – HAVEN 3)
The HAVEN 3 study was a randomised, multicentre, open-label, phase III clinical study in 152 adult and adolescent males (aged ≥12 years and >40 kg) with severe haemophilia A without FVIII inhibitors who previously received either episodic ("on demand") or prophylactic treatment with FVIII. Patients received subcutaneous Hemlibra, 3 mg/kg once weekly for the first four weeks followed by either 1.5 mg/kg once weekly (Arms A and D) or 3 mg/kg every two weeks (Arm B) thereafter, or no prophylaxis (Arm C). Patients in Arm C could switch to Hemlibra (3 mg/kg every two weeks) after completing at least 24 weeks without prophylaxis. For Arms A and B dose up- titration to 3 mg/kg weekly was allowed after 24 weeks for patients who experienced two or more qualified bleeds (i.e., spontaneous and clinically significant bleeds occurring at steady state). Arm D patients could up-titrate after the second qualifying bleed. At the time of the primary analysis, five patients underwent up-titration of their maintenance dose.
Eighty-nine patients previously treated with episodic ("on demand") FVIII were randomized in a 2:2:1 ratio to receive Hemlibra either once weekly (Arm A; N=36), every two weeks (Arm B; N = 35) or no prophylaxis (Arm C; N=18), with stratification by prior 24-week bleed rate (<9 or ≥9). Sixty- three patients previously treated with prophylactic FVIII were enrolled into Arm D to receive Hemlibra (1.5 mg/kg once weekly).
The primary objective of the study was to evaluate in patients previously treated with episodic FVIII the efficacy of prophylactic Hemlibra weekly (Arm A) or every two weeks (Arm B) compared to no prophylaxis (Arm C) based on the number of bleeds requiring treatment with coagulation factors (see Table 4). Other objectives of the study included evaluation of the randomised comparison of Arms A or B and Arm C for the efficacy of Hemlibra prophylaxis in reducing the number of all bleeds, spontaneous bleeds, joint bleeds, and target joint bleeds (see Table 4), as well as assessing patient treatment preference using a preference survey.
The efficacy of Hemlibra prophylaxis was also compared with previous prophylactic FVIII treatment (Arm D) in patients who had participated in a non-interventional study (NIS) prior to enrollment (see Table 5). Only patients from the NIS were included in this comparison, because bleed and treatment data were collected with the same level of granularity as used in HAVEN 3. The NIS is an observational study with the main objective of capturing detailed clinical data on the bleeding episodes and haemophilia medicinal product use of patients with haemophilia A outside of an interventional study setting.
Patients (aged ≥12 years old) with haemophilia A with FVIII inhibitors (Study BH29884 – HAVEN 1)
The HAVEN 1 study was a randomised, multicentre, open-label clinical study in 109 adolescent and adult males (aged ≥12 years old) with haemophilia A with FVIII inhibitors who had previously received either episodic or prophylactic treatment with bypassing agents (aPCC and rFVIIa). In the study, patients received weekly Hemlibra prophylaxis (Arms A, C, and D) — 3 mg/kg once weekly for four weeks followed by 1.5 mg/kg once weekly thereafter — or no prophylaxis (Arm B). Patients randomised to Arm B could switch to Hemlibra prophylaxis after completing at least 24 weeks without prophylaxis. Dose up-titration to 3 mg/kg once weekly was allowed after 24 weeks on Hemlibra prophylaxis for patients who experienced two or more qualified bleeds (i.e., spontaneous and verified clinically significant bleeds occurring at steady state). At the time of the primary analysis, two patients underwent up-titration of their maintenance dose to 3 mg/kg once weekly.
Fifty-three patients previously treated with episodic ("on-demand") bypassing agents were randomised in a 2:1 ratio to receive Hemlibra prophylaxis (Arm A) or no prophylaxis (Arm B), with stratification by prior 24-week bleed rate (<9 or ≥9).
Forty-nine patients previously treated with prophylactic bypassing agents were enrolled in Arm C to receive Hemlibra prophylaxis. Seven patients previously treated with episodic ("on-demand") bypassing agents who had participated in the NIS prior to enrolment but were unable to enroll in HAVEN 1 prior to the closure of Arms A and B were enrolled in Arm D to receive Hemlibra prophylaxis.
The primary objective of the study was to evaluate, among patients previously treated with episodic ("on-demand") bypassing agents, the treatment effect of weekly Hemlibra prophylaxis compared with no prophylaxis (Arm A vs. Arm B) on the number of bleeds requiring treatment with coagulation factors over time (minimum of 24 weeks or date of discontinuation) (see Table 6). Other secondary objectives of the randomised comparison of Arms A and B were the efficacy of weekly Hemlibra prophylaxis in reducing the number of all bleeds, spontaneous bleeds, joint bleeds and target joint bleeds (see Table 6), as well as assessing patients' health-related quality of life (HRQoL) and health status (see Tables 10 and 11). The mean exposure time (+SD) for all patients on study was 21.38 weeks (12.01). For each treatment arm, the mean exposure times (+SD) were 28.86 weeks (8.37) for Arm A, 8.79 (3.62) for Arm B, 21.56 (11.85) for Arm C and 7.08 (3.89) for Arm D. One patient in Arm A withdrew from study prior to initiation of Hemlibra.
The study also evaluated the efficacy of weekly Hemlibra prophylaxis compared with previous episodic (on-demand) and prophylactic bypassing agents (separate comparisons) in patients who had participated in the NIS prior to enrolment (Arms A and C, respectively) (see Table 7).
Patients (aged ≥12 years old) with haemophilia A with or without FVIII inhibitors (Study BO39182 – HAVEN 4)
Hemlibra was investigated in a single arm, multicentre, phase III clinical study in 41 adult and adolescent males (aged ≥12 years and > 40 kg) who have haemophilia A with FVIII inhibitors or severe haemophilia A without FVIII inhibitors who previously received either episodic ("on demand") or prophylactic treatment with bypassing agents or FVIII. Patients received Hemlibra prophylaxis – 3 mg/kg once weekly for four weeks followed by 6 mg/kg every four weeks thereafter. The primary objective of the study was to evaluate the efficacy of Hemlibra prophylaxis given every four weeks in maintaining adequate bleed control, based on treated bleeds. Other objectives were to evaluate the clinical efficacy of Hemlibra prophylaxis on all bleeds, treated spontaneous bleeds, treated joint bleeds and treated target joint bleeds (see Table 8). Patient treatment preference was also assessed using a preference survey.
Patients (all ages) with mild or moderate haemophilia A without FVIII inhibitors (Study BO41423 – HAVEN 6)
The HAVEN 6 study was a multicentre, open-label, single-arm phase III clinical study in 71 emicizumab-treated patients (all ages) with mild (n=20 [28.2%]) or moderate (n=51 [71.8%]) haemophilia A without FVIII inhibitors for whom prophylaxis was indicated, as assessed by the investigator. Most patients were male (69 patients [97.2%]), and 2 were female (2.8%). At study entry, 34 patients (47.9%) were on episodic and 37 patients (52.1%) were on prophylactic treatment with FVIII. Patients received subcutaneous Hemlibra 3 mg/kg once weekly for the first four weeks followed by patient preference for one of the following maintenance regimens, from week 5: 1.5 mg/kg once weekly (n=24 [33.8%]), 3 mg/kg every two weeks (n = 39 [54.9%]) or 6 mg/kg every four weeks (n=8 [11.3%]). Dose up-titration to 3 mg/kg weekly was allowed after 24 weeks for patients who experienced two or more qualified bleeds (i.e., spontaneous and clinically significant bleeds occurring at steady state). At the time of interim analysis, no patients underwent up-titration of their maintenance dose.
The primary efficacy objective of the study was to evaluate the efficacy of Hemlibra prophylaxis based on the number of bleeds requiring treatment with coagulation factors over time (i.e., bleed rate of treated bleeds, see Table 9). Other objectives were to evaluate the efficacy of Hemlibra prophylaxis based on the number of all bleeds, spontaneous bleeds, joint bleeds, and target joint bleeds over time, as well as assessing patient reported HRQoL using the Comprehensive Assessment Tool of Challenges in Haemophilia (CATCH) questionnaire over time.
Efficacy results
HAVEN 3
The efficacy results of Hemlibra prophylaxis compared with no prophylaxis with respect to rate of treated bleeds, all bleeds, treated spontaneous bleeds, treated joint bleeds, and treated target joint bleeds are shown in Table 4.
Table 4. HAVEN 3 study: Annualised Bleed Rate for Hemlibra prophylaxis arm versus no prophylaxis arm in patients ≥12 years of age without FVIII inhibitors:
| Endpoint | Arm C: No prophylaxis (N=18) | Arm A: Hemlibra 1.5 mg/kg weekly (N=36) | Arm B: Hemlibra 3 mg/kg every 2 weeks (N=35) |
|---|---|---|---|
| Treated bleeds | |||
| ABR (95% CI) | 38.2 (22.9; 63.8) | 1.5 (0.9; 2.5) | 1.3 (0.8; 2.3) |
| % reduction (RR), p-value | NA | 96% (0.04), < 0.0001 | 97% (0.03), < 0.0001 |
| % patients with 0 bleeds (95% CI) | 0.0 (0.0; 18.5) | 55.6 (38.1; 72.1) | 60.0 (42.1; 76.1) |
| Median ABR (IQR) | 40.4 (25.3; 56.7) | 0 (0; 2.5) | 0 (0; 1.9) |
| All bleeds | |||
| ABR (95% CI) | 47.6 (28.5; 79.6) | 2.5 (1.6; 3.9) | 2.6 (1.6; 4.3) |
| % reduction (RR), p-value | NA | 95% (0.05 <0.0001 | 94% (0.06), <0.0001 |
| % patients with 0 bleeds (95% CI) | 0 (0.0:18.5) | 50 (32.9; 67.1) | 40 (23.9; 57.9) |
| Treated spontaneous bleeds | |||
| ABR (95% CI) | 15.6 (7.6; 31.9) | 1.0 (0.5; 1.9) | 0.3 (0.1; 0.8) |
| % reduction (RR), p-value | NA | 94% (0.06), <0.0001 | 98% (0.02), <0.0001 |
| % patients with 0 bleeds (95% CI) | 22.2 (6.4; 47.6) | 66.7 (49.0; 81.4) | 88.6 (73.3; 96.8) |
| Treated joint bleeds | |||
| ABR (95% CI) | 26.5 (14.67; 47.79) | 1.1 (0.59; 1.89) | 0.9 (0.44; 1.67) |
| % reduction (RR), p-value | NA | 96% (0.04), <0.0001 | 97% (0.03), <0.0001 |
| % patients with 0 bleeds (95% CI) | 0 (0; 18.5) | 58.3 (40.8; 74.5) | 74.3 (56.7; 87.5) |
| Treated target joint bleeds | |||
| ABR (95% CI) | 13.0 (5.2; 32.3) | 0.6 (0.3; 1.4) | 0.7 (0.3; 1.6) |
| % reduction (RR), p-value | NA | 95% (0.05), <0.0001 | 95% (0.05), <0.0001 |
| % patients with 0 bleeds (95% CI) | 27.8 (9.7; 53.5) | 69.4 (51.9; 83.7) | 77.1 (59.9; 89.6) |
Rate ratio, and confidence interval (CI) come from negative binomial regression (NBR) model and p-value from Stratified Wald test, comparing bleed rate between specified arms.
Arm C: includes no prophylaxis period only.
Bleed definitions adapted based on ISTH criteria.
Treated bleeds = bleeds treated with FVIII
All bleeds = bleeds treated and not treated with FVIII.
Includes data before up-titration only, for patients whose dose was up-titrated.
Patients exposed to emicizumab started with a loading dose of 3 mg/kg/week for 4 weeks.
ABR = Annualised Bleed Rate; CI = confidence interval; RR = rate ratio; IQR = interquartile range, 25th percentile to 75th percentile, NA = Not Applicable
In the HAVEN 3 clinical study intra-patient analysis, Hemlibra prophylaxis resulted in a statistically significant (p<0.0001) reduction (68 %) in bleed rate for treated bleeds compared with previous FVIII prophylaxis collected in the NIS prior to enrollment (see Table 5).
Table 5. HAVEN 3 study: Intra-patient comparison of Annualised Bleed Rate (treated bleeds) with Hemlibra prophylaxis versus previous FVIII prophylaxis:
| Endpoint | Arm D NIS: Previous FVIII prophylaxis (N=48) | Arm D: Hemlibra 1.5 mg/kg weekly (N=48) |
|---|---|---|
| Median efficacy period (weeks) | 30.1 | 33.7 |
| Treated bleeds | ||
| ABR (95% CI) | 4.8 (3.2; 7.1) | 1.5 (1; 2.3) |
| % reduction (RR), p-value | 68% (0.32), <0.0001 | |
| % patients with zero bleeds (95% CI) | 39.6 (25.8; 54.7) | 54.2 (39.2; 68.6) |
| Median ABR (IQR) | 1.8 (0; 7.6) | 0 (0; 2.1) |
Rate ratio and confidence interval (CI) comes from negative binomial regression (NBR) model and p-value from Stratified Wald test, comparing ABR between specified arms.
Intra-patient comparator data from the NIS. Only patients who participated in the NIS and in study HAVEN 3 are included.
Includes data before up-titration only, for patients whose dose was up-titrated.
Treated bleeds = bleeds treated with FVIII. Bleed definitions adapted based on ISTH criteria. ABR = Annualised Bleed Rate; CI = confidence interval; RR = rate ratio; IQR=interquartile range, 25th percentile to 75th percentile
Although a higher adherence was observed with emicizumab prophylaxis than with prior FVIII prophylaxis, no difference in ABR in patients with ≥80% or <80% compliant doses on FVIII prophylaxis according to standard label requirements could be identified (data to be interpreted with caution due to small sample sizes).
Due to the short half-life of FVIII, no carryover effect is assumed after its discontinuation.
Only the first five emicizumab doses had to be administered under supervision to ensure safety and injection technique proficiency. Similar to FVIII prophylaxis, self-administration at home was allowed for all subsequent emicizumab doses.
All patients were treated by haemophilia experts who confirmed that adequate FVIII prophylaxis was administered to patients included in the intra-patient comparison, supporting equivalent usual prophylaxis care across sites and patients.
HAVEN 1
The efficacy results of Hemlibra prophylaxis compared with no prophylaxis with respect to rate of treated bleeds, all bleeds, treated spontaneous bleeds, treated joint bleeds, and treated target joint bleeds are shown in Table 6.
Table 6. HAVEN 1: Annualised Bleed Rate with Hemlibra prophylaxis arm versus no prophylaxis arm in patients ≥12 years of age with FVIII inhibitors:
| Endpoint | Arm B: no prophylaxis | Arm A: 1.5 mg/kg Hemlibra weekly |
|---|---|---|
| N=18 | N=35 | |
| Treated bleeds | ||
| ABR (95% CI) | 23.3 (12.33; 43.89) | 2.9 (1.69; 5.02) |
| % reduction (RR), p-value | 87% (0.13), < 0.0001 | |
| % patients with 0 bleeds (95% CI) | 5.6 (0.1; 27.3) | 62.9 (44.9; 78.5) |
| Median ABR (IQR) | 18.8 (12.97;35.08) | 0 (0; 3.73) |
| All bleeds | ||
| ABR (95% CI) | 28.3 (16.79; 47.76) | 5.5 (3.58; 8.60) |
| % reduction (RR), p-value | 80% (0.20), < 0.0001 | |
| % patients with 0 bleeds (95% CI) | 5.6 (0.1; 27.3) | 37.1 (21.5; 55.1) |
| Treated spontaneous bleeds | ||
| ABR (95% CI) | 16.8 (9.94; 28.30) | 1.3 (0.73; 2.19) |
| % reduction (RR), p-value | 92% (0.08), < 0.0001 | |
| % patients with 0 bleeds (95% CI) | 11.1 (1.4; 34.7) | 68.6 (50.7; 83.1) |
| Treated joint bleeds | ||
| ABR (95% CI) | 6.7 (1.99; 22.42) | 0.8 (0.26; 2.20) |
| % reduction (RR), p-value | 89% (0.11), 0.0050 | |
| % patients with 0 bleeds (95% CI) | 50.0 (26.0; 74.0) | 85.7 (69.7; 95.2) |
| Treated target joint bleeds | ||
| ABR (95% CI) | 3.0 (0.96; 9.13) | 0.1 (0.03; 0.58) |
| % reduction (RR), p-value | 95% (0.05), 0.0002 | |
| % patients with 0 bleeds (95% CI) | 50.0 (26.0; 74.0) | 94.3 (80.8; 99.3) |
Rate ratio, and confidence interval (CI) come from negative binomial regression (NBR) model and p-value from Stratified Wald test, comparing bleed rate between specified arms.
Arm B: includes no prophylaxis period only.
Bleed definitions adapted based on ISTH criteria.
Treated bleeds = bleeds treated with bypassing agents.
All bleeds = bleeds treated and not treated with bypassing agents.
Includes data before up-titration only, for patients whose dose was up-titrated.
Patients exposed to emicizumab started with a loading dose of 3 mg/kg/week for 4 weeks.
ABR = Annualised Bleed Rate; CI = confidence interval; RR = rate ratio; IQR = interquartile range, 25th percentile to 75th percentile.
Table 7. HAVEN 1: Intra-patient comparison of Annualised Bleed Rate (treated bleeds) with Hemlibra prophylaxis versus previous bypassing agent prophylaxis (NIS patients):
| Endpoint | Arm CNIS: previous bypassing agent prophylaxis | Arm C: Hemlibra 1.5 mg/kg weekly |
|---|---|---|
| N=24 | N=24 | |
| Treated bleeds | ||
| ABR (95% CI) | 15.7 (11.08; 22.29) | 3.3 (1.33; 8.08) |
| % patients with 0 bleeds (95% CI) | 12.5 (2.7; 32.4) | 70.8 (48.9; 87.4) |
| Median ABR (IQR) | 12.0 (5.73; 24.22) | 0.0 (0.00; 2.23) |
| % reduction (RR), p-value | 79% (0.21), 0.0003 | |
Rate ratio and confidence interval (CI) comes from negative binomial regression (NBR) model and p-value
from Stratified Wald test, comparing ABR between specified arms.
Intra-patient comparator data from the NIS.
Only patients who participated in the NIS and in study HAVEN 1 are included.
Includes data before up-titration only, for patients whose dose was up-titrated.
Treated bleeds = bleeds treated with bypassing agents.
Bleed definitions adapted based on ISTH criteria.
ABR= Annualised Bleed Rate; CI= confidence interval; RR= rate ratio; IQR=interquartile range, 25 th
percentile to 75 th percentile
Although a higher adherence was observed with emicizumab prophylaxis than with prior bypassing agent
(BPA) prophylaxis, no difference in ABR in patients with ≥ 80% or < 80% compliant doses on BPA
prophylaxis according to standard label requirements could be identified (data to be interpreted with caution
due to small sample sizes).
Due to the short half-life of bypassing agents, no carryover effect is assumed after its discontinuation.
Only the first five emicizumab doses had to be administered under supervision to ensure safety and injection
technique proficiency. Similar to BPA prophylaxis, self-administration at home was allowed for all subsequent
emicizumab doses.
HAVEN 4
Primary analysis efficacy results of Hemlibra prophylaxis every four weeks with respect to rate of treated bleeds, all bleeds, treated spontaneous bleeds, treated joint bleeds, and treated target joint bleeds are shown in Table 8. Forty one patients ≥12 years old were evaluated for efficacy with a median observation time of 25.6 weeks (range: 24.1-29.4).
Table 8. HAVEN 4: Annualised Bleed Rate with Hemlibra prophylaxis in patients ≥12 years of age with or without FVIII inhibitors:
| Hemlibra 6mg/kg Q4W | |||
|---|---|---|---|
| Endpoints | aABR (95% CI) | bMedian ABR (IQR) | % Zero Bleeds (95% CI) |
| N | 41 | 41 | 41 |
| Treated bleeds | 2.4 (1.4; 4.3) | 0.0 (0.0; 2.1) | 56.1 (39.7; 71.5) |
| All bleeds | 4.5 (3.1; 6.6) | 2.1 (0.0; 5.9) | 29.3 (16.1; 45.5) |
| Treated spontaneous bleeds | 0.6 (0.3; 1.5) | 0.0 (0.0; 0.0) | 82.9 (67.9;92.8) |
| Treated joint bleeds | 1.7 (0.8; 3.7) | 0.0 (0.0; 1.9) | 70.7 (54.5; 83.9) |
| Treated target joint bleeds | 1.0 (0.3; 3.3) | 0.0 (0.0; 0.0) | 85.4 (70.8; 94.4) |
a Calculated with negative binomial regression (NBR) model
b Calculated ABR
Bleed definitions adapted based on ISTH criteria
Treated bleeds: bleeds treated with FVIII or rFVIIa
All bleeds: bleeds treated and not treated with FVIII or rFVIIa
Patients exposed to emicizumab started with a loading dose of 3mg/kg/week for 4 weeks.
ABR = Annualised Bleed Rate, CI = confidence interval; IQR = interquartile range; 25th percentile to 75th percentile ; Q4W=once every four week prophylaxis
HAVEN 6 (interim analysis)
Fifty-one patients with moderate haemophilia A aged 2 to 56 years old were evaluated for efficacy with a median observation time of 30.4 weeks (range: 17.4 - 61.7). Interim efficacy results of Hemlibra prophylaxis in patients with moderate haemophilia A (see section 4.1) with respect to rate of treated bleeds, all bleeds, treated spontaneous bleeds, treated joint bleeds, and treated target joint bleeds are shown in Table 9.
Table 9. HAVEN 6: Annualised Bleed Rate with Hemlibra prophylaxis in patients with moderate haemophilia A without FVIII inhibitors:
| cHemlibra 1.5 mg/kg QW, 3 mg/kg Q2W or 6 mg/kg Q4W | |||
|---|---|---|---|
| Endpoints | aABR (95% CI) | bMedian ABR (IQR) | % Zero Bleeds (95% CI) |
| N | 51 | 51 | 51 |
| Treated bleeds | 0.9 [0.43; 1.89] | 0.0 [0.00; 0.00] | 78.4 [64.7; 88.7] |
| All bleeds | 2.6 [1.81; 3.81] | 1.7 [0.00; 3.90] | 43.1 [29.3; 57.8] |
| Treated spontaneous bleeds | 0.1 [0.03; 0.30] | 0.0 [0.00; 0.00] | 94.1 [83.8; 98.8] |
| Treated joint bleeds | 0.3 [0.10; 0.84] | 0.0 [0.00; 0.00] | 90.2 [78.6; 96.7] |
| Treated target joint bleeds | 0.1 [0.02; 0.26] | 0.0 [0.00; 0.00] | 96.1 [86.5; 99.5] |
a Calculated with negative binomial regression (NBR) model
b Calculated ABR
Bleed definitions adapted based on ISTH criteria
Treated bleeds: bleeds treated with FVIII.
All bleeds: bleeds treated and not treated with FVIII.
Patients exposed to emicizumab started with a loading dose of 3 mg/kg/week for 4 weeks.
ABR = Annualised Bleed Rate, CI = confidence interval; IQR = interquartile range; 25th percentile to 75th percentile; QW = once every week prophylaxis; Q2W = once every two weeks prophylaxis; Q4W = once every four weeks prophylaxis
c 1.5 mg/kg QW (n=16); 3 mg/kg Q2W (n=30); 6 mg/kg Q4W (n=5)
Health-related outcome measures
The HAVEN clinical studies evaluated HRQoL and health-status using clinical outcome assessment measures. HAVEN 1 and 2 used the Haemophilia-Specific Quality of Life (Haem-A-QoL) questionnaire for adults (>18 years) and its adolescent version (Haemo-QoL-SF, for 8 to <18 years), respectively, for which the Physical Health Score (i.e. painful swellings, presence of joint pain, pain with movement, difficulty walking far and needing more time to get ready) and Total Score (summary of all scores) were protocol defined endpoints of interest. HAVEN 2 additionally used the Adapted InhibQoL with Aspects of Caregiver Burden questionnaire to obtain caregiver-report of HRQoL in paediatric patients <12 years. HAVEN 6 assessed HRQoL in adult and paediatric patients, as well as caregivers of paediatric patients, using the Comprehensive Assessment Tool of Challenges in Haemophilia (CATCH) questionnaire. The domains of risk perception and impact of haemophilia on daily activities, social activities, recreational activities, and work/school, as well as preoccupation and treatment burden were examined. To measure change in health status, the Index Utility Score (IUS) and the Visual Analog Scale (VAS) from the EuroQoL Five-Dimension Five-Levels Questionnaire (EQ-5D-5L) were examined.
HAVEN 1 health-related outcomes
In this study baseline Total Scores (mean = 41.14 and 44.58, respectively) and Physical Health scale scores (mean = 52.41 and 57.19, respectively) were similar for Hemlibra prophylaxis and no prophylaxis. Table 10 provides a summary of the comparison between the Hemlibra prophylaxis arm (Arm A) and the no prophylaxis arm (Arm B) on the Haem-A-QoL Total Score and Physical Health scale after 24 weeks of treatment adjusting for baseline. Weekly Hemlibra prophylaxis showed a statistically significant and clinically meaningful improvement compared with no prophylaxis in the pre-specified endpoints of Haem-A-QoL Physical Health Scale score at the Week 25 assessment.
Table 10. HAVEN 1: Change in Haem-A-QoL Physical Health and Total score with Hemlibra prophylaxis versus no prophylaxis in patients ≥18 years with FVIII inhibitors:
| Haem-A-QoL at week 25 | Arm B: no prophylaxis (N=14) | Arm A: Hemlibra 1.5 mg/kg weekly (N=25) |
|---|---|---|
| Physical health score (range 0 to 100) | ||
| Adjusted mean | 54.17 | 32.61 |
| Difference in adjusted means (95% CI) | 21.55 (7.89, 35.22) | |
| p-value | 0.0029 | |
| Total score (range 0 to 100) | ||
| Adjusted mean | 43.21 | 29.2 |
| Difference in adjusted means (95% CI) | 14.01 (5.56, 22.45) | |
Arm B: includes no prophylaxis period only.
Includes data before up-titration only, for patients whose dose was up-titrated.
Patients exposed to emicizumab started with a loading dose of 3 mg/kg/week for 4 weeks.
Haem-A_QoL scales range from 0 to 100; lower scores are reflective of better HRQoL.
Clinically meaningful difference: Total score: 7 points; Physical Health: 10 points. Analyses are based on data from individuals who provided responses at both baseline and Week 25 assessments.
Table 11. HAVEN 1: EQ-5D-5L scores in patients ≥12 years at week 25:
| EQ-5D-5L scores after 24 weeks | Arm B: no prophylaxis (N=16) | Arm A: Hemlibra 1.5 mg/kg weekly (N=29) |
|---|---|---|
| Visual Analogue Scale | ||
| Adjusted mean | 74.36 | 84.08 |
| Difference in adjusted means (95% CI) | -9.72 (-17.62, -1.82) | |
| Index Utility Score | ||
| Adjusted mean | 0.65 | 0.81 |
| Difference in adjusted means (95% CI) | -0.16 (-0.25, -0.07) | |
Arm B: includes no prophylaxis period only.
Includes data before up-titration only, for patients whose dose was up-titrated.
Patients exposed to emicizumab started with a loading dose of 3 mg/kg/week for 4 weeks.
Higher scores indicate better quality of life.
Clinically meaningful difference: VAS: 7 points, Index Utility Score: 0.07 points
Analyses are based on data from individuals who provided responses at both baseline and Week 25 assessments.
HAVEN 6 health-related outcomes
In HAVEN 6, HRQoL for patients of all ages with moderate haemophilia A was evaluated at week 25 based on the CATCH questionnaire. The CATCH questionnaire (version 1.0) is a validated instrument that assesses the effect of haemophilia and its treatment. Different versions of the questionnaire exist for adult patients, paediatric patients and caregivers of paediatric patients. Health-related quality of life on Hemlibra prophylaxis remained generally stable, with improvement in the treatment burden domain of CATCH consistently observed across respondent groups.
Paediatric population
Paediatric patients (age <12 years old, or 12 to 17 years old weighing <40 kg) with haemophilia A with FVIII inhibitors (Study BH29992 – HAVEN 2)
Hemlibra weekly prophylaxis was evaluated in a single-arm, multicentre, open-label clinical study in paediatric patients (age <12 years old, or 12 to 17 years old weighing <40 kg) with haemophilia A with FVIII inhibitors. Patients received Hemlibra prophylaxis at 3 mg/kg once weekly for the first 4 weeks followed by 1.5 mg/kg once weekly thereafter.
The study evaluated the pharmacokinetics (PK), safety, and efficacy including the efficacy of weekly Hemlibra prophylaxis compared with previous episodic and prophylactic bypassing agent treatment in patients who had participated in the NIS prior to enrolment (intra-patient comparison).
Efficacy results
HAVEN 2 (interim analysis)
At the time of the interim analysis, efficacy was evaluated in 59 patients who were <12 years old and had been receiving weekly Hemlibra prophylaxis for at least 12 weeks, including four patients aged <2 years old, 17 patients aged 2 to <6 years, 38 patients aged 6 to <12 years old. Annualised bleed rate and percent of patients with zero bleeds were calculated (see Table 12). The median observation time for these patients was 29.6 weeks (range: 18.4 to 63.0 weeks).
Table 12. HAVEN 2: Overview of efficacy (interim analysis):
| Endpoint | aABR (95% CI) bN=59 | cMedian ABR (IQR) bN=59 | % Zero Bleeds (95% CI) bN=59 |
|---|---|---|---|
| Treated bleeds | 0.3 (0.1; 0.5) | 0 (0; 0) | 86.4 (75; 94) |
| All bleeds | 3.8 (2.2; 6.5) | 0 (0; 3.4) | 55.9 (42.4; 68.8) |
| Treated spontaneous bleeds | 0 (0; 0.2) | 0 (0; 0) | 98.3 (90.9; 100) |
| Treated joint bleeds | 0.2 (0.1; 0.4) | 0 (0; 0) | 89.8 (79.2; 96.2) |
| Treated target joint bleeds | 0.1 (0; 0.7) | 0 (0; 0) | 96.6 (88.3; 99.6) |
ABR = annualised bleed rate; CI = confidence interval; IQR = interquartile range, 25th percentile to 75th percentile
a Calculated with negative binomial regression (NBR) model.
b Efficacy data from treated patients aged < 12 years who had been on study HAVEN 2 for at least 12 weeks (N = 59), as the study aimed to primarily investigate treatment effect based on age.
c Calculated ABR
Bleed definitions adapted based on ISTH criteria.
Treated bleeds: bleeds treated with bypassing agents.
All bleeds: bleeds treated and not treated with bypassing agents.
Patients exposed to emicizumab started with a loading dose of 3 mg/kg/week for 4 weeks.
In the intra-patient analysis, Hemlibra weekly prophylaxis resulted in a clinically meaningful reduction (98 %) in treated bleed rate in 18 paediatric patients who had at least 12 weeks of Hemlibra prophylaxis compared to their bleed rate collected in the NIS prior to enrolment (Table 13).
Table 13. HAVEN 2: Intra-patient comparison of Annualised Bleed Rate (treated bleeds) with Hemlibra prophylaxis versus previous bypassing agent prophylaxis:
| Endpoint | Previous bypassing agent treatment* (N=18) | Hemlibra prophylaxis (N=18) |
|---|---|---|
| Treated bleeds | ||
| ABR (95% CI) | 19.8 (15.3; 25.7) | 0.4 (0.15; 0.88) |
| % reduction (RR) | 98% (0.02) | |
| % patients with zero bleeds (95% CI) | 5.6 (0.1; 27.3) | 77.8 (52.4; 93.6) |
| Median ABR (IQR) | 16.2 (11.49; 25.78) | 0 (0; 0) |
* Previous prophylactic treatment for 15 of the 18 patients; previous episodic (on-demand) treatment for 3 subject
Rate ratio and confidence interval (CI) comes from negative binomial regression (NBR) model and p-value from Stratified Wald test, comparing ABR between specified arms.
Intra-patient comparator data from the NIS.
Only patients who participated in the NIS and in study HAVEN 2 are included.
Bleed definitions adapted based on ISTH criteria.
Treated bleeds: bleeds treated with bypassing agents.
Patients exposed to emicizumab started with a loading dose of 3 mg/kg/week for 4 weeks.
ABR = Annualised Bleed Rate; CI = confidence interval; RR = rate ratio; IQR = interquartile range, 25th percentile to 75th percentile
Although a higher adherence was observed with emicizumab prophylaxis than with prior bypassing agent (BPA) prophylaxis, no difference in ABR in patients with ≥80% or <80% compliant doses on BPA prophylaxis according to standard label requirements could be identified (data to be interpreted with caution due to small sample sizes).
Due to the short half-life of bypassing agents, no carryover effect is assumed after its discontinuation.
Only the first five emicizumab doses had to be administered under supervision to ensure safety and injection technique proficiency. Similar to BPA prophylaxis, self-administration at home was allowed for all subsequent emicizumab doses.
Paediatric health-related outcomes results
HAVEN 2 health-related outcomes
In HAVEN 2, HRQoL for patients aged ≥8 to <12 years was evaluated at week 25 based on the Haemo-QoL-SF questionnaire for children (see Table 14). The Haemo-QoL-SF is a valid and reliable measure of HRQoL. HRQoL for patients aged <12 years was also evaluated at week 25 based on the Adapted InhibQoL with Aspects of Caregiver Burden questionnaire completed by caregivers (see Table 14). The Adapted InhibQoL is a valid and reliable measure of HRQoL.
Table 14. HAVEN 2: Change from baseline to week 25 in the Physical Health score of patients (<12 years of age) following treatment with Hemlibra prophylaxis as reported by patients and caregivers:
| Haemo-QoL-SF | |
| Physical health score (range 0 to 100)a | |
| Mean baseline score (95% CI) (n=18) | 29.5 (16.4 – 42.7) |
| Mean change from baseline (95% CI) (n=15) | -21.7 (-37.1 - -6.3) |
| Adapted InhibQoL with aspects of caregiver burden | |
| Physical health score (range 0 to 100)a | |
| Mean baseline score (95% CI) (n=54) | 37.2 (31.5 – 42.8) |
| Mean change from baseline (95% CI) (n=43) | -32.4 (-38.6 - -26.2) |
a Lower scores (negative change scores) are reflective of better functioning.
Analyses are based on data from individuals who provided responses at both baseline and Week 25 assessments.
There is limited experience with bypassing agent or FVIII use during surgeries and procedures. Bypassing agent or FVIII use during surgeries and procedures was determined by the investigator.
In the event of breakthrough bleeding, patients receiving emicizumab prophylaxis should be managed with available therapies. For bypassing agent guidance refer to section 4.4.
Immunogenicity
As with all therapeutic proteins, there is the potential for an immune response in patients treated with emicizumab. A total of 739 patients were tested for anti-emicizumab antibodies in the pooled clinical studies. Thirty-six patients (4.9%) tested positive for anti-emicizumab antibodies. In 19 patients (2.6%), anti-emicizumab antibodies were neutralising in vitro. Of these 19 patients, the neutralising anti-emicizumab antibodies did not have a clinically meaningful impact on the pharmacokinetics or efficacy of Hemlibra in 15 patients, while decreased emicizumab plasma concentrations were observed in four patients (0.5%). One patient (0.1%) with neutralising anti-emicizumab antibodies and decreased emicizumab plasma concentrations experienced loss of efficacy after five weeks of treatment and discontinued Hemlibra. Overall, the safety profile of Hemlibra was similar between those patients with anti-emicizumab antibodies (including neutralising antibodies) and those without (see sections 4.4 and 4.8).
Elderly population
Use of Hemlibra in patients aged 65 and over with haemophilia A is supported by studies HAVEN 1, HAVEN 3, HAVEN 4 and HAVEN 6. Based on limited data, there is no evidence to suggest a difference in efficacy or safety in patients aged 65 years or above.
Pharmacokinetic properties
The pharmacokinetics of emicizumab was determined via non-compartmental analysis in healthy subjects and using a population pharmacokinetic analysis on a database composed of 389 patients with haemophilia A.
Absorption
Following subcutaneous administration in haemophilia A patients, the absorption half-life was 1.6 days.
Following multiple subcutaneous administrations of 3 mg/kg once weekly for the first 4 weeks in haemophilia A patients, mean (±SD) trough plasma concentrations of emicizumab achieved 52.6±13.6 μg/mL at Week 5.
The predicted mean (±SD) Ctrough, and Cmax and ratios of Cmax/Ctrough at steady-state for the recommended maintenance doses of 1.5 mg/kg once weekly, 3 mg/kg every two weeks or 6 mg/kg every four weeks are shown in Table 15.
Table 15. Mean (± SD) steady-state emicizumab concentrations:
| Maintenance dose | |||
|---|---|---|---|
| Parameters | 1.5 mg/kg QW | 3 mg/kg Q2W | 6 mg/kg Q4W |
| Cmax,ss (µg/mL) | 54.9 ± 15.9 | 58.1 ± 16.5 | 66.8 ± 17.7 |
| Cavg,ss (µg/mL) | 53.5 ± 15.7 | 53.5 ± 15.7 | 53.5 ± 15.7 |
| Ctrough,ss (µg/mL) | 51.1 ± 15.3 | 46.7 ± 16.9 | 38.3 ± 14.3 |
| Cmax/Ctrough ratio | 1.08 ± 0.03 | 1.26 ± 0.12 | 1.85 ± 0.46 |
Cavg,ss = average concentration at steady state; Cmax,ss = maximum plasma concentration at steady state; Ctrough,ss = trough concentration at steady state; QW = once weekly; Q2W = every two weeks; Q4W = every four weeks.
Pharmacokinetic parameters derived from the population PK model.
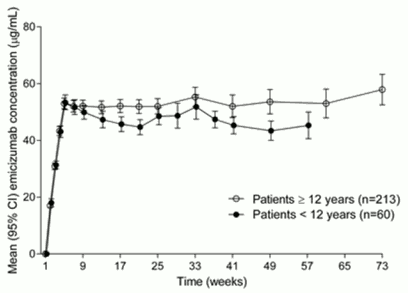
Similar PK profiles were observed following once weekly dosing (3 mg/kg/week for 4 weeks followed by 1.5 mg/kg/week) in adults/adolescents (≥12 years) and children (<12 years) (see Figure 1).
Figure 1. Mean (±95% CI) plasma emicizumab concentration versus time profiles for patients ≥12 years (studies HAVEN 1 and HAVEN 3) compared with patients <12 years (study HAVEN 2):
In healthy subjects, the absolute bioavailability following subcutaneous administration of 1 mg/kg was between 80.4% and 93.1% depending on the injection site. Similar pharmacokinetic profiles were observed following subcutaneous administration in the abdomen, upper arm, and thigh. Emicizumab can be administered interchangeably at these anatomical sites (see section 4.2).
Distribution
Following a single intravenous dose of 0.25 mg/kg emicizumab in healthy subjects, the volume of distribution at steady state was 106 mL/kg (i.e. 7.4 L for a 70-kg adult).
The apparent volume of distribution (V/F), estimated from the population PK analysis, in haemophilia A patients following multiple subcutaneous doses of emicizumab was 10.4 L.
Metabolism
The metabolism of emicizumab has not been studied. IgG antibodies are mainly catabolised by lysosomal proteolysis and then eliminated from or reused by the body.
Elimination
Following intravenous administration of 0.25 mg/kg in healthy subjects, the total clearance of emicizumab was 3.26 mL/kg/day (i.e. 0.228 L/d for a 70-kg adult) and the mean terminal half-life was 26.7 days.
Following single subcutaneous injection in healthy subjects, the elimination half-life was approximately 4 to 5 weeks.
Following multiple subcutaneous injections in haemophilia A patients, the apparent clearance was 0.272 L/day and the elimination apparent half-life was 26.8 days.
Dose linearity
Emicizumab exhibited dose-proportional pharmacokinetics in patients with haemophilia A after the first dose of Hemlibra over a dose range from 0.3 to 6 mg/kg. The exposure (Cavg,ss) of multiple doses is comparable between 1.5 mg/kg every week, 3mg/kg every 2 weeks and 6mg/kg dose every 4 weeks.
Special populations
Paediatric
The effect of age on the pharmacokinetics of emicizumab was assessed in a population pharmacokinetic analysis which included 5 infants (≥1 month to <2 years), 55 children (less than 12 years) and 50 adolescents (12 to <18 years) with haemophilia A. Age did not affect the pharmacokinetics of emicizumab in paediatric patients.
Elderly
The effect of age on the pharmacokinetics of emicizumab was assessed in a population pharmacokinetic analysis which included thirteen subjects aged 65 years and older (no subjects were older than 77 years of age). Relative bioavailability decreased with older age, but no clinically important differences were observed in the pharmacokinetics of emicizumab between subjects <65 years and subjects ≥65 years.
Race
Population pharmacokinetics analyses in patients with haemophilia A showed that race did not affect the pharmacokinetics of emicizumab. No dose adjustment is required for this demographic factor.
Gender
Data in female patients are too limited for conclusion.
Renal impairment
No dedicated studies of the effect of renal impairment on the pharmacokinetics of emicizumab have been conducted.
Most of the patients with hemophilia A in the population pharmacokinetic analysis had normal renal function (N=332; creatinine clearance [CLcr] ≥90 mL/min) or mild renal impairment (N=27; CLcr of 60-89 mL/min). Mild renal impairment did not affect the pharmacokinetics of emicizumab. There are limited data available on the use of Hemlibra in patients with moderate renal impairment (only 2 patients with CLcr of 30-59 mL/min) and no data in patients with severe renal impairment. The impact of moderate and severe renal impairment on the pharmacokinetics of emicizumab cannot be concluded.
Emicizumab is a monoclonal antibody and is cleared via catabolism rather than renal excretion and a change in dose is not expected to be required for patients with renal impairment.
Hepatic impairment
No dedicated studies on the effect of hepatic impairment on the pharmacokinetics of emicizumab have been conducted. Most of the patients with haemophilia A in the population pharmacokinetic analysis had normal hepatic function (bilirubin and AST ≤ ULN, N=300) or mild hepatic impairment (bilirubin ≤ ULN and AST > ULN or bilirubin from 1.0 to 1.5 × ULN and any AST, N=51). Only 6 patients had moderate hepatic impairment (1.5 × ULN < bilirubin ≤3 × ULN and any AST). Mild hepatic impairment did not affect the pharmacokinetics of emicizumab (see section 4.2). The safety and efficacy of emicizumab have not been specifically tested in patients with hepatic impairment. Patients with mild and moderate hepatic impairment were included in clinical trials. No data are available on the use of Hemlibra in patients with severe hepatic impairment.
Emicizumab is a monoclonal antibody and cleared via catabolism rather than hepatic metabolism and a change in dose is not expected to be required for patients with hepatic impairment.
Other special populations
Modelling shows that less frequent dosing in patients with hypoalbuminemia and low body weight for their age results in lower emicizumab exposures; simulations indicate that these patients would still benefit from clinically meaningful bleed control. No patients with such characteristics were enrolled in clinical trials.
Preclinical safety data
Preclinical data reveal no special hazards for humans based on studies of acute and repeated dose toxicity, including safety pharmacology endpoints and endpoints for reproductive toxicity.
Fertility
Emicizumab did not cause any changes in the reproductive organs of male or female cynomolgus monkeys up to the highest tested dose of 30 mg/kg/week (equivalent to 11 times the human exposure at the highest dose of 3 mg/kg/week, based on AUC).
Teratogenicity
No data are available with respect to potential side effects of emicizumab on embryo-foetal development.
Injection site reactions
Reversible hemorrhage, perivascular mononuclear cell infiltration, degeneration/necrosis of subcutis and swelling of endothelium in the subcutis was noted in animals after subcutaneous injection.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.