HEPAGAM B Solution for infusion Ref.[10823] Active ingredients: Human hepatitis B immunoglobulin
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
HepaGam B provides passive immunization for individuals exposed to the hepatitis B virus, by binding to the surface antigen and reducing the rate of hepatitis B infection13-16.
12.3. Pharmacokinetics
The pharmacokinetic profile of HepaGam B has been evaluated in two 84-day clinical trials in which 70 healthy subjects received an intramuscular injection of 0.06 milliliter per kilogram of HepaGam B. The mean peak concentrations (Cmax) in both studies were comparable and occurred within 4-5 days of administration. Both studies demonstrated mean elimination half-lives (t½) following IM administration of 22 to 25 days. The mean clearance rate was 0.21 to 0.24 liter per day and the volume of distribution was approximately 7.5 liter. Thus, HepaGam B demonstrates pharmacokinetic parameters similar to those reported by Scheiermann and Kuwert17.
The maximum concentration of anti-HBs achieved by HepaGam B was consistent with that of two other licensed comparator Hepatitis B Immune Globulin (Human) products6. Comparability of pharmacokinetics between HepaGam B and a commercially available hepatitis B immune globulin product administered IM indicates that comparable efficacy of HepaGam B should be inferred.
14. Clinical Studies
14.1 Clinical Trials in Liver Transplant Patients
A clinical trial examined the effectiveness of HepaGam B in the prevention of hepatitis B recurrence following liver transplantation. The study was a multi-center, open-labeled, superiority study involving HBsAg-positive/HBeAg-negative liver transplant patients. The study included two arms, an active treatment group of patients enrolled to receive the described dosing regimen of HepaGam B starting during transplant and continuing over the course of a year, and a retrospective untreated control group of historical patients with data gathered by chart review.
There were 27 liver transplant patients who received HepaGam B and 14 retrospective untreated control patients. The patients in both groups were HBsAg-positive/HBeAg-negative liver transplant patients who met similar entry criteria, had similar medical history and had similar status at transplant based on MELD and/or ChildPugh-Turcotte scores.
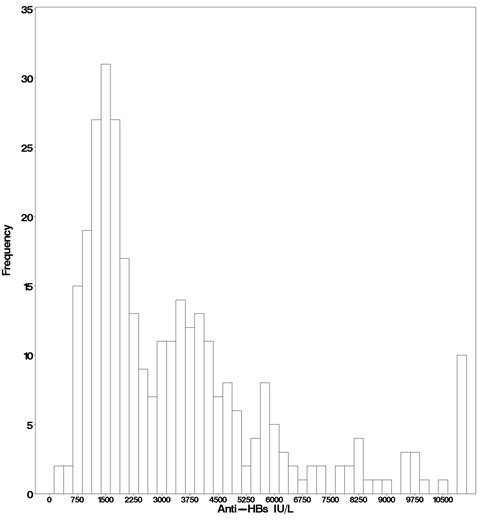
In the active treatment group, HepaGam B intravenous doses of 35 milliliters were initiated during transplant according to the regimen identified in Table 1 [see Dosage and Administration (2.1)]. As a result of the targeted potency of 550 IU per milliliter at the time of manufacture [see Dosage Forms and Strengths (3)], the 35 milliliter doses of HepaGam B used in this study actually contained between 17,000 and 23,000 IU anti-HBs. These 35 milliliter doses consistently yielded anti-HBs trough levels >500 IU per liter (99% of all anti-HBs levels were >500 IU per liter; see Figure 1). Patients received HepaGam B doses diluted with 50 mL of saline.
Figure 1. Frequency Histogram of Trough anti-HBs Levels more than 30 days after Transplant:
Values below the target trough were only observed in the 2 patients with HBV recurrence who had anti-HBs levels <150 IU per liter at the time of seroconversion.
For the efficacy endpoint of the proportion of patients with HBV recurrence (HBsAg positive and/or HBeAg positive after 4 weeks post-OLT), a significant treatment effect was observed. As summarized in Table 5, HBV recurrence was seen in 2/24 or 8.3% of HepaGam B patients compared to 12/14 or 86% of retrospective untreated control patients (see Table 5). Two of the HepaGam B patients who died within 28 days post-transplant were excluded from all efficacy analyses, but included for safety analyses. The deaths were not HBV or study drug related.
Table 5. Results of Study HB-005 for the Prevention of Hepatitis B Recurrence Following Liver Transplantation:
| HepaGam B | Retrospective Untreated Control | P-value (Fisher's Exact Test) | |
|---|---|---|---|
| HBV Recurrence Proportion, % (95% confidence interval) | 8.3 (0.1-27.0) | 85.7 (57.2-98.2) | <0.001 |
The conclusion that HepaGam B monotherapy post-OLT is effective at preventing HBV recurrence post-OLT is further supported by the secondary endpoints of time to recurrence, survival, anti-HBs levels, biochemical markers of liver inflammation, and liver biopsy. Time to recurrence for the HepaGam B treatment group was 358 days for two HBV recurrent patients. In comparison, the retrospective untreated control patients had a median time to recurrence of 88 days with a 95% confidence interval of 47 to 125 days. Survival calculations showed that 96% (23/24) of patients in the active treatment group survived for at least 1 year post-OLT compared to 43% (6/14) retrospective control patients. The endpoints for HBV recurrence were supported by an observed drop in anti-HBs levels, elevated liver function tests, and abnormal liver biopsy result at the time of recurrence.
HepaGam B is recommended in patients who have no or low levels of viral replication at the time of liver transplantation. The clinical trial evaluating HepaGam B in liver transplant patients selected patients with no or low replication status only. HepaGam B therapy has not been evaluated in combination with antiviral therapy post-transplantation.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.