HEPLISAV B Solution for injection in pre-filled syringe Ref.[28057] Active ingredients: Hepatitis B, purified antigen
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Dynavax GmbH, Eichsfelder Strasse 11, D-40595 Düsseldorf, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Vaccines, Viral Vaccines, Hepatitis Vaccine
ATC code: J07BC01
Mechanism of action
HEPLISAV B is comprised of recombinant hepatitis B surface antigen and the CpG 1018 adjuvant, which is a 22-mer immunostimulatory sequence oligonucleotide.
HEPLISAV B induces specific antibodies against HBsAg (anti-HBs).
The biological actions of CpG 1018 are exerted locally at the injection site and draining lymph nodes. The adjuvant CpG 1018 component of HEPLISAV B has the following effects: (1) activates plasmacytoid dendritic cells (pDCs) through the pattern recognition receptor Toll-like receptor 9; (2) converts pDCs into highly efficient antigen-presenting cells that present the processed HBsAg to CD4+ T cells; and, (3) promotes Th1 T-cell differentiation through the production of IFN-alpha and IL-12. This activation results in a high and sustained antibody response, likely due to the rapid generation of large numbers of anti-HBs-secreting plasmacytes and HBsAg-specific memory B and T cells.
Immune responses to HEPLISAV B
No efficacy trials were conducted due to the application of the well-established immune correlate of protection to the immune response (anti-HBs concentration ≥10 mIU/ml correlates with protection against HBV infection). The immunogenicity of HEPLISAV B was evaluated in 3 randomized, active controlled, observer-blinded, multicentre phase 3 clinical trials (HBV-10 with 3:1 randomisation, HBV-16 with 4:1 randomisation, and HBV-23 with 2:1 randomisation) including 9365 adults aged 18 to 70 years given HEPLISAV B, and 3867 adults given the comparator hepatitis B vaccine (Engerix-B 20 mcg HBsAg). HEPLISAV B was given as a 2-dose schedule at 0 and 1 month and Engerix-B was given using a 3-dose schedule at 0, 1, and 6 months.
Baseline characteristics were balanced between the treatment arms for age, sex, race, ethnicity, and body mass index (BMI). In the pooled analysis including all 3 trials, the mean age was 49.3 and 49.4 in the HEPLISAV B arm and Engerix-B arms, respectively and there were 50.8% and 51.5% female participants who received HEPLISAV B and Engerix-B, respectively.
The trials evaluated the seroprotection rates (SPR: percentage of vaccinated persons whose anti-HBs antibody levels were ≥10 mIU/ml after vaccination) after the second dose of HEPLISAV B compared to after the third dose of Engerix-B. The SPR and peak geometric mean concentration (GMC) after a 2-dose schedule of HEPLISAV B were statistically significantly higher than after a 3-dose schedule of Engerix-B (lower bound of the 95% confidence interval of the difference in SPRs between HEPLISAV B and Engerix-B was greater than 0%; lower bound of the 95% confidence interval of the ratio of GMCs between HEPLISAV B and Engerix-B was greater than 1.0) in all 3 trials (Table 1, Table 2).
Table 1. Comparison of Seroprotection Rates Between HEPLISAV B and Engerix-B at Peak Weeks in Pooled Trials HBV-23, HBV-16 and HBV-10 (mITT Population):
| HEPLISAV B | Engerix-B | Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| N | n | SPR () (95 CI) | N | n | SPR () (95 CI) | (HEPLISAV B – Engerix-B) (95% CI) | ||
| 8701 | 8327 | 95.7 (95.3 – 96.1) | 3643 | 2898 | 79.5 (78.2 – 80.8) | 16.2 (14.8 – 17.6) | ||
N = number of evaluable subjects; n = number of seroprotected subjects; SPR = Seroprotection Rate, CI = confidence interval.
Seroprotection is defined as anti-HBs ≥10 mIU/mL.
Peak week comparison is for HEPLISAV B at Week 24 and Engerix-B at Week 28.
The confidence intervals on seroprotection rates are calculated using the two-sided Clopper-Pearson method.
The confidence interval on the difference between treatment groups is calculated using the Miettinen and Nurminen method without stratification.
Table 2. Comparison of Anti-HBs Geometric Mean Concentrations at Peak Weeks Between HEPLISAV B and Engerix-B in Pooled Trials HBV-23, HBV-16 and HBV-10 (mITT Population):
| HEPLISAV B | Engerix-B | GMC Ratio | |||
|---|---|---|---|---|---|
| N | GMC (95% CI) | N | GMC (95% CI) | (HEPLISAV B / Engerix-B) (95% CI) | |
| 8701 | 329.1 (317.1 – 341.5) | 3642 | 262.3 (236.4 – 291.1) | 1.3 (1.1 – 1.4) | |
Peak week for HEPLISAV B is Week 24. Peak week for Engerix-B is Week 28
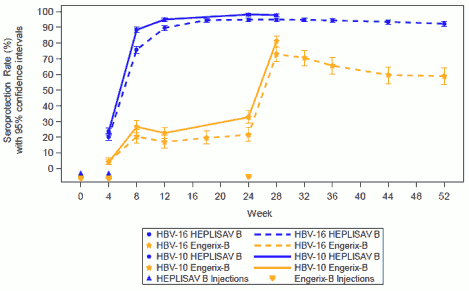
SPR results were collected at each study visit in two of the pivotal trials, HBV-10 (week 4 to 28) and HBV-16 (week 4 to 52). HEPLISAV B induced significantly higher SPRs than Engerix-B across all study visits in both studies (Figure 1).
Figure 1. Seroprotection Rates by Visit in Trials HBV-16 and HBV-10 (Per Protocol Population):
In all three trials, SPRs induced by HEPLISAV B were statistically significantly higher than those induced by Engerix-B in older adults, men, obese individuals, smokers and subjects with type II diabetes mellitus (Table 3).
Table 3. Comparison of Seroprotection Rates Between HEPLISAV B and Engerix-B at Peak Weeks by Category in Pooled Trials HBV-23, HBV-16 and HBV-10 (mITT Population):
| Category | HEPLISAV B | Engerix-B | Difference | ||||
|---|---|---|---|---|---|---|---|
| N | n | SPR () (95 CI) | N | n | SPR () (95 CI) | (HEPLISAV B – Engerix-B) (95% CI) | |
| All subjects | 8701 | 8327 | 95.7 (95.3 – 96.1) | 3643 | 2898 | 79.5 (78.2 – 80.8) | 16.2 (14.8 – 17.6) |
| Age Group (years) | |||||||
| 18 – 29 | 527 | 526 | 99.8 (98.9 – 100.0) | 211 | 196 | 92.9 (88.5 – 96.0) | 6.9 (4.1 – 11.2) |
| 30 – 39 | 1239 | 1227 | 99.0 (98.3 – 99.5) | 545 | 483 | 88.6 (85.7 – 91.2) | 10.4 (7.9 – 13.4) |
| 40 – 49 | 2377 | 2310 | 97.2 (96.4 – 97.8) | 963 | 771 | 80.1 (77.4 – 82.5) | 17.1 (14.6 – 19.8) |
| 50 – 59 | 2712 | 2578 | 95.1 (94.2 – 95.8) | 1120 | 872 | 77.9 (75.3 – 80.3) | 17.2 (14.7 – 19.8) |
| ≥60 | 1846 | 1686 | 91.3 (90.0 – 92.6) | 804 | 576 | 71.6 (68.4 – 74.7) | 19.7 (16.4 – 23.1) |
| Sex | |||||||
| Male | 4274 | 4055 | 94.9 (94.2 – 95.5) | 1765 | 1361 | 77.1 (75.1 – 79.1) | 17.8 (15.7 – 19.9) |
| Female | 4427 | 4272 | 96.5 (95.9 – 97.0) | 1878 | 1537 | 81.8 (80.0 – 83.6) | 14.7 (12.9 – 16.5) |
| BMI Stratum | |||||||
| <30 kg/m2 | 4904 | 4728 | 96.4 (95.9 – 96.9) | 2069 | 1756 | 84.9 (83.3 – 86.4) | 11.5 (10.0 – 13.2) |
| ≥30 kg/m2 | 3789 | 3591 | 94.8 (94.0 – 95.5) | 1570 | 1140 | 72.6 (70.3 – 74.8) | 22.2 (19.9 – 24.5) |
| Smoking Status | |||||||
| Smoker | 2634 | 2538 | 96.4 (95.6 – 97.0) | 1130 | 852 | 75.4 (72.8 – 77.9) | 21.0 (18.4 – 23.6) |
| Non-smoker | 6067 | 5789 | 95.4 (94.9 – 95.9) | 2513 | 2046 | 81.4 (79.8 – 82.9) | 14.0 (12.4 – 15.7) |
| Type 2 Diabetes Status and Age Group (Years) | |||||||
| With T2D | |||||||
| 20 – 39 | 38 | 37 | 97.4 (86.2 – 99.9) | 16 | 12 | 75.0 (47.6 – 92.7) | 22.4 (5.1 – 47.5) |
| 40 – 49 | 163 | 151 | 92.6 (87.5 – 96.1) | 67 | 49 | 73.1 (60.9 – 83.2) | 19.5 (9.2 – 31.7) |
| 50 – 59 | 334 | 303 | 90.7 (87.1 – 93.6) | 160 | 108 | 67.5 (59.7 – 74.7) | 23.2 (15.6 – 31.4) |
| ≥60 | 377 | 320 | 84.9 (80.9 – 88.3) | 165 | 97 | 58.8 (50.9 – 66.4) | 26.1 (17.9 – 34.5) |
BMI = body mass index; CI = confidence interval; N = number of evaluable subjects; n = number of seroprotected subjects;
SPR = Seroprotection Rate; T2D = type 2 diabetes.
Seroprotection is defined as anti-HBs = 10 mIU/mL.
Peak week comparison is for HEPLISAV B at Week 24 and Engerix-B at Week 28.
The confidence intervals on seroprotection rates are calculated using the two-sided Clopper-Pearson method.
The confidence interval on the difference between treatment groups is calculated using the Miettinen and Nurminen method without stratification.
Haemodialysis
In a phase 3, randomized, open-label, multicentre study of 116 adult subjects with haemodialysis-dependent chronic kidney disease (CKD) who were non-responders to previous hepatitis B vaccination, participants received a 1-dose booster regimen of HEPLISAV B or Fendrix, or a double booster dose of Engerix-B.
Week 4 SPR in the HEPLISAV B group (42.1% n=16/38) was higher than the SPR in the Engerix-B group (18.9%, n=7/37) and the Fendrix group (29.3%, n=12/41). At Week 12, the SPR was 24.3% (n=9/37) in the HEPLISAV B group, 13.9% (n=11/41) in the Engerix-B group, and 26.8% (n=11/41) in the Fendrix group.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with HEPLISAV B in one or more subsets of the paediatric population for the prevention of hepatitis B virus infection (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
The pharmacokinetic properties of the hepatitis B surface antigen used in HEPLISAV B have not been assessed.
Renal Impairment
The CpG 1018 adjuvant is cleared from plasma within 24 hours in renally-impaired adults after a single dose of 3000 micrograms. Dose adjustment is not required.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies consisting of single-dose and repeat-dose toxicity (including local tolerance), and reproductive and developmental toxicity.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.