HYPERRAB Solution for injection Ref.[10828] Active ingredients: Human rabies immunoglobulin
Source: FDA, National Drug Code (US) Revision Year: 2019
12.1. Mechanism of Action
HYPERRAB provides immediate, passive, rabies virus neutralizing antibody coverage until the previously unvaccinated patient responds to rabies vaccine by actively producing antibodies.(1)
12.2. Pharmacodynamics
The usefulness of prophylactic rabies antibody in preventing rabies in humans when administered immediately after exposure was dramatically demonstrated in a group of persons bitten by a rabid wolf in Iran.(7,8) Similarly, beneficial results were later reported from the U.S.S.R.(9) Studies coordinated by WHO (World Health Organization) helped determine the optimal conditions under which antirabies serum of equine origin and rabies vaccine can be used in man.(10-13) These studies showed that antirabies serum can interfere to a variable extent with the active immunity induced by the vaccine, but could be minimized by booster doses of vaccine after the end of the usual dosage series.
Preparation of rabies immune globulin of human origin with adequate potency was reported by Cabasso et al.(14) In carefully controlled clinical studies, this globulin was used in conjunction with rabies vaccine of duck-embryo origin (DEV).(14,15) These studies determined that a human globulin dose of 20 IU/kg of rabies antibody, given simultaneously with the first DEV dose, resulted in amply detectable levels of passive rabies antibody 24 hours after injection in all recipients. The injections produced minimal, if any, interference with the subject's endogenous antibody response to DEV.
Subsequently, human diploid cell rabies vaccines (HDCV) prepared from tissue culture fluids containing rabies virus have received substantial clinical evaluation in Europe and the United States.(14-22) In a study in adult volunteers, the administration of Rabies Immune Globulin (Human) did not interfere with antibody formation induced by HDCV when given in a dose of 20 IU per kilogram body weight simultaneously with the first dose of vaccine.(21)
12.3. Pharmacokinetics
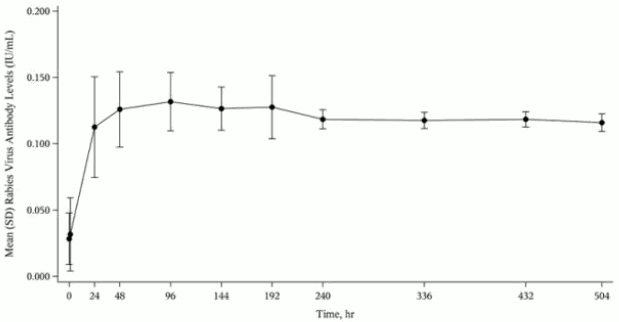
In a clinical study of 12 healthy human subjects receiving a 20 IU/kg intramuscular dose of HYPERRAB detectable passive rabies neutralizing antibody was present by 24 hours and persisted through the 21 day follow-up evaluation period. Figure 1 shows the mean levels of rabies virus antibodies in IU/mL across the 21 day evaluation period and indicates that the titer remains stable during this period. This level of passive rabies neutralizing antibody is similar to that reported in the literature for administration of human rabies immune globulin, and is clinically important because it provides interim protection until the host immune response to rabies vaccine produces definitive protective titers of neutralizing rabies antibody (therefore the rabies vaccine series is also essential).(23-24)
Figure 1. Mean (Standard Deviation) Rabies Virus Neutralizing Antibody Levels (IU/mL) versus Time following a Single 20 IU/kg Dose of HYPERRAB (300 I/mL) by Intramuscular Injection:
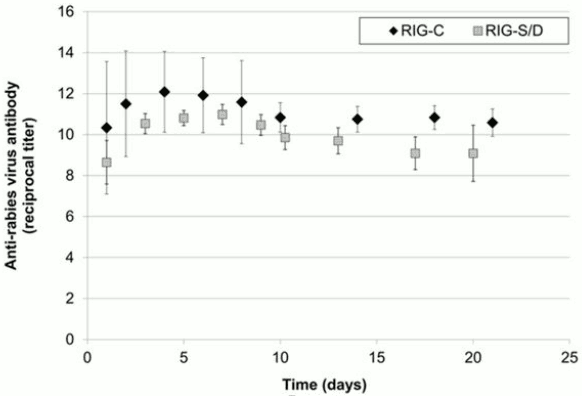
The previous formulation, HYPERRAB S/D, was studied in 8 healthy subjects over 21 days. As with the new formulation, rabies neutralizing antibody was present by 24 hours and persisted through the 21 day follow up period (Figure 2).
Figure 2. Reciprocal of Anti-Rabies Virus Neutralizing Antibody Titer Following a Single 20 IU/kg Dose of HYPERRAB (300 IU/mL; RIG-C) or HYPERRAB S/D (150 IU/mL; RIG-S/D) Product (mean [standard deviation]):
14. Clinical Studies
HYPERRAB was administered to a total of 20 healthy adult subjects in two clinical trials [see Clinical Pharmacology (12.3)]. A single intramuscular dose of 20 IU/kg HYPERRAB (12 subjects) or HYPERRAB S/D (8 subjects) was administered and rabies neutralizing antibody titers were monitored in serum for 21 days. Administration of both HYPERRAB formulations resulted in detectable titers of neutralizing antibodies to the rabies virus that persisted throughout the 21 day study period (Figure 2).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.