HYPERTET Solution for injection Ref.[10844] Active ingredients: Tetanus immunoglobulin
Source: FDA, National Drug Code (US) Revision Year: 2020
3. Indications and Usage
HyperTET is indicated for prophylaxis against tetanus following injury in patients whose immunization is incomplete or uncertain (see below). It is also indicated, although evidence of effectiveness is limited, in the regimen of treatment of active cases of tetanus.(8,9,16)
A thorough attempt must be made to determine whether a patient has completed primary vaccination. Patients with unknown or uncertain previous vaccination histories should be considered to have had no previous tetanus toxoid doses. Persons who had military service since 1941 can be considered to have received at least one dose, and although most of them may have completed a primary series of tetanus toxoid, this cannot be assumed for each individual. Patients who have not completed a primary series may require tetanus toxoid and passive immunization at the time of wound cleaning and debridement.(3)
The following table is a summary guide to tetanus prophylaxis in wound management.
Guide to Tetanus Prophylaxis in Wound Management(3):
| History of Tetanus Immunization (Doses) | Clean, Minor Wounds | All Other Wounds* | ||
|---|---|---|---|---|
| Td† | TIG‡ | Td | TIG | |
| Uncertain or less than 3 | Yes | No | Yes | Yes |
| 3 or more§ | No¶ | No | No# | No |
* Such as, but not limited to, wounds contaminated with dirt, feces, soil, and saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns and frostbite.
† Adult type tetanus and diphtheria toxoids. If the patient is less than 7 years old, DT or DTP is preferred to tetanus toxoid alone. For persons ≥7 years of age, Td is preferred to tetanus toxoid alone. (see Dosage and Administration)
‡ Tetanus Immune Globulin (Human).
§ If only three doses of fluid tetanus toxoid have been received, a fourth dose of toxoid, preferably an adsorbed toxoid, should be given.
¶ Yes if more than 10 years since the last dose.
# Yes if more than 5 years since the last dose. (More frequent boosters are not needed and can accentuate side effects).
10. Dosage and Administration
Routine prophylactic dosage schedule
Adults and children 7 years and older: HyperTET, 250 units should be given by deep intramuscular injection (see PRECAUTIONS). At the same time, but in a different extremity and with a separate syringe, Tetanus and Diphtheria Toxoids Adsorbed (For Adult Use) (Td) should be administered according to the manufacturer's package insert. Adults with uncertain histories of a complete primary vaccination series should receive a primary series using the combined Td toxoid. To ensure continued protection, booster doses of Td should be given every 10 years.(3)
Children less than 7 years old: In small children the routine prophylactic dose of HyperTET may be calculated by the body weight (4.0 units/kg). However, it may be advisable to administer the entire contents of the syringe of HyperTET (250 units) regardless of the child's size, since theoretically the same amount of toxin will be produced in the child's body by the infecting tetanus organism as it will in an adult's body. At the same time but in a different extremity and with a different syringe, Diphtheria and Tetanus Toxoids and Pertussis Vaccine Adsorbed (DTP) or Diphtheria and Tetanus Toxoids Adsorbed (For Pediatric Use) (DT), if pertussis vaccine is contraindicated, should be administered per the manufacturer's package insert.
Note: The single injection of tetanus toxoid only initiates the series for producing active immunity in the recipient. The physician must impress upon the patient the need for further toxoid injections in 1 month and 1 year. Without such, the active immunization series is incomplete. If a contraindication to using tetanus toxoid-containing preparations exists for a person who has not completed a primary series of tetanus toxoid immunization and that person has a wound that is neither clean nor minor, only passive immunization should be given using tetanus immune globulin.(3) See table under INDICATIONS AND USAGE.
Available evidence indicates that complete primary vaccination with tetanus toxoid provides long lasting protection ≥10 years for most recipients. Consequently, after complete primary tetanus vaccination, boosters-even for wound management-need be given only every 10 years when wounds are minor and uncontaminated. For other wounds, a booster is appropriate if the patient has not received tetanus toxoid within the preceding 5 years. Persons who have received at least two doses of tetanus toxoid rapidly develop antibodies.(3) The prophylactic dosage schedule for these patients and for those with incomplete or uncertain immunity is shown on the table in INDICATIONS AND USAGE.
Since tetanus is actually a local infection, proper initial wound care is of paramount importance. The use of antitoxin is adjunctive to this procedure. However, in approximately 10% of recent tetanus cases, no wound or other breach in skin or mucous membrane could be implicated.(18)
Treatment of active cases of tetanus
Standard therapy for the treatment of active tetanus including the use of HyperTET must be implemented immediately. The dosage should be adjusted according to the severity of the infection.(8,9)
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. They should not be used if particulate matter and/or discoloration are present.
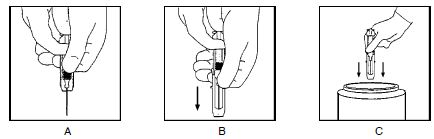
HyperTET is supplied with a syringe and an attached UltraSafe Needle Guard for your protection and convenience. Please follow instructions below for proper use of syringe and UltraSafe Needle Guard.
Directions for Syringe Usage
- Remove the prefilled syringe from the package. Lift syringe by barrel, not by plunger.
- Twist the plunger rod clockwise until the threads are seated.
- With the needle shield secured on the syringe tip, push the plunger rod forward a few millimeters to break any friction seal between the stopper and the glass syringe barrel.
- Remove the needle shield and expel air bubbles. [Do not remove the needle shield to prepare the product for administration until immediately prior to the anticipated injection time.]
- Proceed with hypodermic needle puncture.
- Aspirate prior to injection to confirm that the needle is not in a vein or artery.
- Inject the medication.
- Keeping your hands behind the needle, grasp the guard with free hand and slide forward toward needle until it is completely covered and guard clicks into place. If audible click is not heard, guard may not be completely activated. (See Diagrams A and B)
- Place entire prefilled glass syringe with guard activated into an approved sharps container for proper disposal. (See Diagram C)
A number of factors could reduce the efficacy of this product or even result in an ill effect following its use. These include improper storage and handling of the product after it leaves our hands, diagnosis, dosage, method of administration, and biological differences in individual patients. Because of these factors it is important that this product be stored properly and that the directions be followed carefully during use.
9. Overdosage
Although no data are available, clinical experience with other immunoglobulin preparations suggests that the only manifestations would be pain and tenderness at the injection site.
12. Storage and handling
Store at 2–8°C (36–46°F). Solution that has been frozen should not be used. Discard unused portion.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.