IDAMYCIN PFS Solution for injection Ref.[50722] Active ingredients: Idarubicin
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
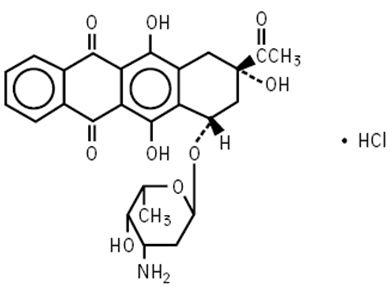
IDAMYCIN PFS Injection contains idarubicin hydrochloride and is a sterile, semi-synthetic, preservative-free solution (PFS) antineoplastic anthracycline for intravenous use. Chemically, idarubicin hydrochloride is 5, 12-Naphthacenedione, 9-acetyl-7-[(3-amino-2,3,6-trideoxy-α-L-lyxohexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxyhydrochloride, (7Scis). The structural formula is as follows:
C26H27NO9 ∙ HCL M.W. 533.96
IDAMYCIN PFS is a sterile, red-orange, isotonic parenteral preservative-free solution, available in 5 mL (5 mg), 10 mL (10 mg) and 20 mL (20 mg) single-dose only vials.
Each mL contains Idarubicin HCL, USP 1 mg (equivalent to 0.93 mg Idarubicin free base) and the following inactive ingredients: Glycerin, USP 25 mg and Water for Injection, USP q.s. Hydrochloric Acid, NF is used to adjust the pH to a target of 3.5.
| How Supplied |
|---|
|
IDAMYCIN PFS Injection (idarubicin hydrochloride injection). Single Dose Cytosafe Vials: Sterile single-dose only, contains no preservative. Discard unused portion. NDC 0013-2576-91 5 mg/5 mL vial (1 mg/mL), single-dose vials. Distributed by: Pfizer Labs, Division of Pfizer Inc., New York, NY 10017 |
Drugs
| Drug | Countries | |
|---|---|---|
| IDAMYCIN | Japan, Mexico, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.