IMCIVREE Solution for injection Ref.[27947] Active ingredients: Setmelanotide
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Rhythm Pharmaceuticals Netherlands B.V., Radarweg 29, 1043NX Amsterdam, Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: anti-obesity preparations, excl. diet products, centrally acting anti-obesity product
ATC code: A08AA12
Mechanism of action
Setmelanotide is a selective MC4 receptor agonist. MC4 receptors in the brain are involved in regulation of hunger, satiety, and energy expenditure. In genetic forms of obesity associated with insufficient activation of the MC4 receptor, setmelanotide is believed to re-establish MC4 receptor pathway activity to reduce hunger and promote weight loss through decreased caloric intake and increased energy expenditure.
Pharmacodynamic effects
Skin pigmentation
Setmelanotide is a selective MC4 receptor agonist with less activity at the melanocortin 1 (MC1) receptor. The MC1 receptor is expressed on melanocytes, and activation of this receptor leads to accumulation of melanin and increased skin pigmentation independently of ultraviolet light (see sections 4.4 and 4.8).
Clinical efficacy and safety
POMC, including PCSK1, deficiency and LEPR deficiency
The safety and efficacy of setmelanotide for the treatment of POMC and LEPR deficiency obesity were established in 2 identically designed, 1-year open-label pivotal studies, each with a double-blind, placebo-controlled withdrawal period:
- Study 1 (RM-493-012) enrolled patients aged 6 years and above with genetically confirmed POMC (including PCSK1) deficiency obesity.
- Study 2 (RM-493-015) enrolled patients aged 6 years and above with genetically confirmed LEPR deficiency obesity.
In both studies, adult patients had a body mass index (BMI) of ≥30 kg/m². Weight in children was ≥95th percentile using growth chart assessment.
Dose titration occurred over a 2- to 12-week period, followed by a 10-week open-label treatment period. Patients who achieved at least a 5 kg weight loss (or at least 5% weight loss if baseline body weight was <100 kg) at the end of the open-label treatment period continued into a double-blind, placebo-controlled, withdrawal period lasting 8 weeks (4-week placebo treatment and 4-week setmelanotide treatment). Following the withdrawal sequence, patients re-initiated active treatment with setmelanotide at the therapeutic dose for up to 32 weeks. Twenty-one patients (10 in Study 1 and 11 in Study 2) have been treated for at least 1 year and are included in the efficacy analyses.
Additional supportive data were gathered in an investigator-led study and an ongoing extension study.
Study 1 (RM-493-012)
In Study 1, 80% of patients with POMC deficiency obesity met the primary endpoint, achieving a ≥10% weight loss after 1 year of treatment with setmelanotide and 50% of patients with POMC deficiency obesity achieved a predefined clinically meaningful ≥25% improvement in hunger score from baseline at 1 year (Table 15).
Statistically significant and clinically meaningful mean percent decreases from baseline for body weight of 25.6% were reported for Study 1. Changes in hunger were assessed using a patient and caregiver questionnaire completed daily for ‘most hunger over the last 24 hours’ at 1 year for patients ≥12 years of age. Statistically significant and clinically meaningful mean percent decreases from baseline for hunger as a weekly average in the last 24 hours of 27.1% were reported for Study 1 (Table 16).
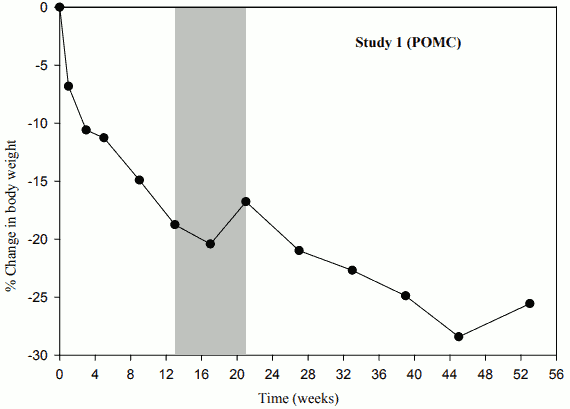
When treatment with setmelanotide was withdrawn in patients who had lost weight during the 10-week open-label period, these patients gained weight (Figure 1) and the mean hunger scores increased over the 4 weeks of placebo treatment.
Table 15. Proportion of patients achieving at least 10% weight loss and the proportion of patients achieving at least 25% improvement in daily hunger from baseline at 1 year in Study 1:
| Parameter | Statistic | |
|---|---|---|
| Patients achieving at least 10% weight loss at 1 year (N=10) | n (%) 90% CI1 P-value2 | 8 (80.0%) (49.31%, 96.32%) <0.0001 |
| Patients achieving at least 25% hunger improvement from baseline at 1 year (N=8) | n (%) 90% CI1 P-value1 | 4 (50.0) (19.29, 80.71) 0.0004 |
Note: The analysis set includes patients who received at least 1 dose of study drug and had at least 1 baseline assessment.
1 From the Clopper-Pearson (exact) method
2 Testing the null hypothesis: proportion=5%
Table 16. Percent change from baseline in weight and hunger at 1 year in Study 1:
| Parameter | Statistic | Body weight (kg) (N=9) | Hunger score1 (N=7) |
|---|---|---|---|
| Baseline | Mean (SD) | 115.0 (37.77) | 8.1 (0.78) |
| Median | 114.7 | 8.0 | |
| Min, Max | 55.9, 186.7 | 7,9 | |
| 1 year | Mean (SD) | 83.1 (21.43) | 5.8 (2.02) |
| Median | 82.7 | 6.0 | |
| Min, Max | 54.5, 121.8 | 3,8 | |
| Percent change from baseline to 1 year (%) | Mean (SD) | -25.6 (9.88) | -27.06 (28.11) |
| Median | -27.3 | -14.29 | |
| Min, Max | -35.6, -2.4 | -72.2, -1.4 | |
| LS Mean | -25.39 | -27.77 | |
| 90% CI | (-28.80, -21.98) | (-40.58, -14.96) | |
| P-value | <0.0001 | 0.0005 |
Note: This analysis includes patients who received at least one dose of study drug, had at least one baseline assessment, and demonstrated ≥5 kg weight loss (or 5% of body weight if weight was <100 kg at baseline) over the 12-week open-label treatment period and proceeded into the double-blind, placebo-controlled withdrawal period.
1 Hunger ranges from 0 to 10 on a Likert-type scale; 0 = not hungry at all and 10 = hungriest possible. Hunger score was captured in a daily diary and was averaged to calculate a weekly score for analysis.
Figure 1. Percent Body Weight Change from Baseline by Visit (Study 1 [N=9]):
Study 2 (RM-493-015)
In Study 2, 46% of patients with LEPR deficiency obesity met the primary endpoint, achieving a ≥10% weight loss after 1 year of treatment with setmelanotide and 73% of patients with LEPR deficiency obesity achieved a predefined clinically meaningful ≥25% improvement in hunger score from baseline at 1 year (Table 17).
Statistically significant and clinically meaningful mean percent decreases from baseline for body weight of 12.5% were reported for Study 2. Changes in hunger were assessed using a patient and caregiver questionnaire completed daily for ‘most hunger over the last 24 hours’ at 1 year for patients ≥12 years of age. Statistically significant and clinically meaningful mean percent decreases from baseline for hunger as a weekly average in the last 24 hours of 43.7% were reported for Study 2 (Table 18).
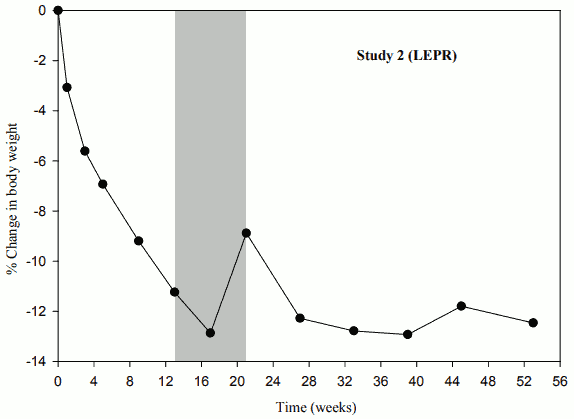
When treatment with setmelanotide was withdrawn in patients who had lost weight during the 10-week open-label period, these patients gained weight (Figure 2) and the mean hunger scores increased over the 4 weeks of placebo treatment.
Table 17. Proportion of patients achieving at least 10% weight loss and the proportion of patients achieving at least 25% improvement in daily hunger from baseline at 1 year in Study 2:
| Parameter | Statistic | |
|---|---|---|
| Patients achieving at least 10% weight loss at 1 year (N=11) | n (%) | 5 (45.5%) |
| 90% CI1 | (19.96%, 72.88%) | |
| P-value2 | 0.0002 | |
| Patients achieving at least 25% hunger improvement from baseline at 1 year (N=11) | n (%) | 8 (72.7) |
| 90% CI1 | (43.56, 92.12) | |
| P-value1 | <0.0001 | |
Note: The analysis set includes patients who received at least 1 dose of study drug and had at least 1 baseline assessment.
1 From the Clopper-Pearson (exact) method
2 Testing the null hypothesis: proportion=5%
Table 18. Percent change from baseline in weight and hunger at 1 year in Study 2:
| Parameter | Statistic | Body weight (kg) (N=7) | Hunger score1 (N=7) |
|---|---|---|---|
| Baseline | Mean (SD) | 131.7 (32.6) | 7.0 (0.77) |
| Median | 120.5 | 7.0 | |
| Min, Max | 89.4, 170.4 | 6,8 | |
| 1 year | Mean (SD) | 115.0 (29.6) | 4.1 (2.09) |
| Median | 104.1 | 3.0 | |
| Min, Max | 81.7, 149.9 | 2,8 | |
| Percent change from baseline to 1 year (%) | Mean (SD) | -12.5 (8.9) | -43.7 (23.69) |
| Median | -15.3 | -52.7 | |
| Min, Max | -23.3, 0.1 | -67,0 | |
| LS Mean | -12.47 | -41.93 | |
| 90% CI | (-16.10, -8.83) | (-54.76, -29.09) | |
| P-value | <0.0001 | <0.0001 |
Note: This analysis includes patients who received at least one dose of study drug, had at least one baseline assessment, and demonstrated ≥5 kg weight loss (or 5% of body weight if weight was <100 kg at baseline) over the 12-week open-label treatment period and proceeded into the double-blind, placebo-controlled withdrawal period.
1 Hunger ranges from 0 to 10 on a Likert-type scale; 0 = not hungry at all and 10 = hungriest possible. Hunger score was captured in a daily diary and was averaged to calculate a weekly score for analysis.
Figure 2. Percent Body Weight Change from Baseline by Visit (Study 2 [N=7]):
Bardet-Biedl Syndrome
Study 3 (RM-493-023)
The safety and efficacy of IMCIVREE for the treatment of patients aged 6 years and older with obesity due to BBS were assessed in a 1-year clinical study with a 14-week placebo-controlled period (Study 3 [RM-493-023]). The study enrolled patients aged 6 years and above with obesity and BBS. Adult patients had a BMI of ≥30 kg/m². Paediatric patients had a BMI ≥97th percentile for age and sex using growth chart assessments.
Eligible patients entered a 14-week, randomized, double-blind, placebo-controlled treatment period (Period 1) followed by a 38-week open-label treatment period (Period 2) in which all patients received setmelanotide. To maintain the blind through Period 2, dose titration to a fixed dose of 3 mg was done during the first 2 weeks of both Period 1 and Period 2. Thirty-two patients have been treated for at least 1 year and are included in the efficacy analyses.
In Study 3, 35.7% of patients with BBS aged ≥12 years and 46.7% of patients with BBS aged ≥18 years met the primary endpoint, achieving a ≥10% weight loss after 1 year of treatment with setmelanotide (Table 19). The effect of IMCIVREE on body weight in patients assessed by the investigator as cognitively impaired was similar to patients who were not cognitively impaired.
In Study 3, ~52 weeks of treatment with setmelanotide resulted in clinically meaningful reductions in BMI Z-scores occurring in 100% of the BBS patients aged <12 years, with consistent results observed in patients ≥12 and <18 years of age. In patients aged <18 years, the mean reduction from baseline in BMI Z-score was 0.75 and the mean reduction from baseline in percent of the 95th percentile for BMI for age and sex was 17.3%.
Patients 12 years and older who were able to self-report their hunger, recorded their daily maximal hunger in a diary, which was then assessed by the Daily Hunger Questionnaire Item 2. Hunger was scored on an 11-point scale from 0 (“not hungry at all”) to 10 (“hungriest possible”). Statistically significant and clinically meaningful mean percent decreases from baseline at 1 year for most/worst hunger of 30.5% were reported for Study 3 (Table 16).
Table 19. Body weight (kg) – proportion of all patients, patients with BBS aged ≥12 years and patients with BBS aged ≥18 years achieving at least 10% weight loss from baseline at 1 year (Study 3 [Full Analysis Set]):
| Parameter | Statistic1 | Patients ≥12 years | Patients ≥18 years |
|---|---|---|---|
| Patients achieving at least 10% weight loss at year 1 | N | 28 | 15 |
| % | 35.7 | 46.7 | |
| 95% CI1 | (18.6, 55.9) | (21.3, 73.4) | |
| P-value | 0.0002 | 0.0003 |
1 Estimated , 95 confidence interval and p-value are based on Rubin’s Rule. P-value is one-sided and compared with alpha=0.025.
Table 20. Daily hunger scores – change from baseline at 1 year in all patients and patients with BBS aged ≥12 years (Study 3 [Full Analysis Set]):
| Timepoint | Statistic | Patients ≥12 years |
|---|---|---|
| Baseline | N | 14 |
| Mean (SD) | 6.99 (1.893) | |
| Median | 7.29 | |
| Min, Max | 4.0, 10.0 | |
| Week 52 | N | 14 |
| Mean (SD) | 4.87 (2.499) | |
| Median | 4.43 | |
| Min, Max | 2.0, 10.0 | |
| Change at week 52 | N | 14 |
| Mean (SD) | -2.12 (2.051) | |
| Median | -1.69 | |
| Min, Max | -6.7, 0.0 | |
| 95% CI1 | -3.31, -0.94 | |
| p-value1 | 0.0010 | |
| % Change at week 52 | N | 14 |
| Mean (SD) | -30.45 (26.485) | |
| Median | -25.00 | |
| Min, Max | -77.0, 0.0 | |
| 95% CI1 | -45.74, -15.16 | |
| p-value1 | 0.0004 |
Abbreviations: CI=confidence interval; Max=maximum; Min=minimum; SD=Standard Deviation.
1 95% CI and p-value are based on Rubin’s Rule; p-value is one-sided.
Note: Baseline is the last assessment prior to initiation of setmelanotide in both studies.
Note: The Daily Hunger Questionnaire is not administered to patients <12 years or to patients with cognitive impairment as assessed by the Investigator.
Supportive of IMCIVREE’s effect on weight loss, there were general numeric improvements in cardiometabolic parameters, such as blood pressure, lipids, glycaemic parameters, and waist circumference.
Paediatric population
The safety and efficacy of setmelanotide for the treatment of patients aged 2 to <6 years with obesity due to POMC or LEPR deficiency or BBS were assessed in a 1-year open-label, non-controlled study 20 (Study 4 [RM-493-033]). The study enrolled patients aged 2 to <6 years with a BMI ≥97th percentile for age and sex using growth chart assessments and a body weight of at least 15 kg at baseline.
Eligible patients entered the study and received setmelanotide. Twelve patients were enrolled in the study and are included in the efficacy analyses. Given the study design and small sample size, efficacy findings require careful consideration.
In Study 4, 85.7% of patients with POMC or LEPR deficiency obesity and 80.0% of the patients with BBS met the primary endpoint, achieving a ≥0.2 BMI Z-score reduction after 1 year of treatment with setmelanotide (Table 21). The mean percent change from baseline to Week 52 in BMI was -25.597% for patients with POMC or LEPR deficiency obesity and -9.719% for patients with BBS (Table 22).
Table 21. BMI Z-score – proportion of all patients, patients with POMC or LEPR deficiency obesity, patients with BBS aged 2 to <6 years achieving at least 0.2 reduction in BMI Z-score from baseline at 1 year (Study 4 [safety population]):
| Parameter | Statistic1 | Patients with POMC or LEPR (n=7) | Patients with BBS (n=5) | Total (N=12) |
|---|---|---|---|---|
| Patients achieving at least 0.2 reduction in BMI Z-score at year 1 | N | 6 | 4 | 10 |
| % | 85.7 | 80.0 | 83.3 | |
| 95% CI1 | (54.1, 100) | (28.4, 99.5) | (58.7, 99.8) |
1 Two-sided 95% CI was calculated using the Clopper-Pearson Method.
In Study 4, ~52 weeks of treatment with setmelanotide resulted in a clinically meaningful reduction in BMI Z-score of -5.185 for patients with POMC or LEPR deficiency obesity and -1.331 for patients with BBS. The mean reduction from baseline in percent of the 95 th percentile for BMI for age and sex was -47.595% for patients with POMC or LEPR deficiency obesity and -14.462% for patients with BBS.
In clinical studies, 44 of the patients treated with setmelanotide were aged 2 to 17 years at baseline (21 patients with POMC, PCSK1 or LEPR deficiency and 33 patients with BBS). Overall, efficacy and safety in these younger patients showed similar trends as seen in older patients studied, with seemingly meaningful decreases in BMI demonstrated. In patients who had not yet completed their growth, a trend towards appropriate progression in pubertal development and increases in height were observed during the study period.
The European Medicines Agency has deferred the obligation to submit the results of studies with setmelanotide in one or more subsets of the paediatric population in treatment of appetite and general nutrition disorders (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
The mean steady state setmelanotide Cmax,ss, AUCtau, and trough concentration for a 3 mg dose administered subcutaneously to otherwise healthy volunteers with obesity (N=6) once daily for 12 weeks were 37.9 ng/ml, 495 h*ng/ml, and 6.77 ng/ml, respectively. Steady-state plasma concentrations of setmelanotide were achieved within 2 days with daily dosing of 1-3 mg setmelanotide. The accumulation of setmelanotide in the systemic circulation during once-daily dosing over 12 weeks was approximately 30%. Setmelanotide AUC and Cmax increased proportionally following multiple-dose subcutaneous administration in the proposed dose range (1-3 mg).
A population PK model comprised of 410 subjects pooled from 11 studies was conducted. These subjects contributed 7087 observations, of which 6847 samples had quantifiable setmelanotide concentrations. The PK data were predominantly from 271 adults and 87 adolescents (aged 12 to <18 years). There were also 41 children aged 6 to <12 years and 11 children aged 2 to <6 years. The population consisted of 166 males and 244 females with ages ranging from 2 to 78 years (mean = 29.7 years) and weights ranging from 17.8 to 246 kg (mean = 113 kg). The pooled population included 329 subjects with POMC, PCSK1, or LEPR deficiency, BBS, or other rare genetic diseases of obesity (80.2%) and 81 subjects without POMC, PCSK1, or LEPR deficiency, BBS, or other rare genetic diseases of obesity (19.8%); all subjects without POMC, PCSK1 or LEPR deficiency, BBS, or other rare genetic diseases of obesity were adults.
Absorption
After subcutaneous injection of setmelanotide, steady-state plasma concentrations of setmelanotide increased slowly, reaching maximum concentrations at a median tmax of 8.0 hours after dosing. The absolute bioavailability following subcutaneous administration of setmelanotide has not been investigated in humans. Estimate of the inter-individual variability (CV%) from the final population PK model was 39.9% (CL/F).
The PK of setmelanotide in patients with BBS was similar to that obtained in the population of patients with POMC, PCSK1, and LEPR deficiency, suggesting the disease state alone does not impact the PK of setmelanotide.
Distribution
The mean apparent volume of distribution of setmelanotide after subcutaneous administration of setmelanotide 3 mg once daily was estimated from the population PK model to be 75.2 L. Setmelanotide binding to human plasma protein is 79.1%.
In vitro experiments indicate that setmelanotide is not a substrate of OATP1B1, OATP1B3, OAT1, OAT3, or OCT2.
In vitro data indicate that setmelanotide is very unlikely a P-gp or BCRP substrate.
Biotransformation
Setmelanotide did not appear to be metabolised by rat, monkey, or human hepatic microsomes or hepatocytes, or kidney microsomes.
Elimination
The effective elimination half-life (t½) of setmelanotide was approximately 11 hours. The total apparent steady state clearance of setmelanotide following subcutaneous administration of 3 mg once daily was estimated from the population PK model to be 7.15 L/h.
Approximately 39% of the administered setmelanotide dose was excreted unchanged in urine during the 24-hour dosing interval following subcutaneous administration of 3 mg once daily.
Linearity/non-linearity
Setmelanotide AUC and Cmax increased approximately linearly with dose following multiple-dose subcutaneous administration in the range of 0.5 mg to 5 mg.
Special populations
Paediatric population
Setmelanotide has been evaluated in paediatric patients (aged 2 to 17 years). Simulations from the population PK analyses suggest slightly higher exposure in younger patients (who also have lower body weight) and provide support for the dosing regimen in patients 2 years and older.
Elderly population
Available data in a small sample of elderly patients suggest no marked changes in setmelanotide exposure with increased age. However, these data are too limited to draw definite conclusions.
Renal impairment
Pharmacokinetic analysis showed a 12%, 26%, and 49% lower clearance (CL/F) of setmelanotide in patients with mild, moderate, and severe renal impairment, respectively, as compared to patients with normal renal function.
POMC, including PCSK1, deficiency and LEPR deficiency
No dose adjustments for patients with mild (estimated glomerular filtration rate [eGFR] of 60-89 ml/min/1.73 m²) or moderate renal impairment (eGFR of 30-59 ml/min/1.73 m²) are needed. Dose adjustments are recommended for patients with severe renal impairment (eGFR 15-29 ml/min/1.73 m²) (see section 4.2). Setmelanotide should not be administered to patients with end-stage renal disease (eGFR <15 ml/min/1.73 m²) (see section 4.2).
Bardet-Biedl Syndrome
No dose adjustments for patients with mild (estimated glomerular filtration rate [eGFR] of 60-89 ml/min/1.73 m²) or moderate renal impairment (eGFR of 30-59 ml/min/1.73 m²) are needed. Dose adjustments are recommended for patients with severe renal impairment (eGFR 15-29 ml/min/1.73 m²) (see section 4.2). Setmelanotide should not be administered to patients with end-stage renal disease (eGFR <15 ml/min/1.73 m²) (see section 4.2).
Hepatic impairment
Setmelanotide is stable in human, rat, and monkey hepatocytes; therefore, a study in patients with hepatic impairment was not conducted. Setmelanotide should not be used in patients with hepatic impairment.
Body weight
Setmelanotide CL/F varied with body weight according to a fixed allometric relationship.
Gender
No clinically significant differences in the pharmacokinetics of setmelanotide were observed based on sex.
5.3. Preclinical safety data
Nonclinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, genotoxicity, carcinogenicity, fertility, teratogenicity, or postnatal development.
A developmental reproduction study in rabbits revealed increases in embryo-foetal resorption and post-implantation loss in pregnant rabbits treated with setmelanotide. These effects were attributed to extreme reductions in maternal food consumption related to the primary pharmacodynamic activity of setmelanotide. Similar reductions in food consumption and related embryo-foetal loss were not observed in a developmental reproduction study in rats. No teratogenic effects were observed in either species.
Dose-related setmelanotide concentrations were observed in milk 2 hours after subcutaneous injection in the pre-weaning phase of a pre- and postnatal development study in rats. No quantifiable setmelanotide concentrations were detected in plasma from nursing pups at any dose.
In contrast to primates, variable cardiovascular effects, such as increased heart rate and blood pressure, were observed in rats and minipigs. The reason underlying those species differences remains unclear. In rat, the dose-dependent effects of setmelanotide on heart rate and blood pressure were linked to an increase in sympathetic tone and they were found to progressively diminish upon repeated daily dosing.
Minimal cytoplasmic vacuolation related to the excipient mPEG-DSPE was observed in the choroid plexus after chronic administration in adult rats and monkeys. Choroid plexus vacuolation was not observed in juvenile rats treated with setmelanotide/mPEG-DSPE from post-natal Days 7 to 55 at 9.5-times the human dose of mPEG-DSPE from 3 mg of setmelanotide on a mg/m²/day basis.
The available carcinogenicity data in Tg.rasH2 mice indicate that setmelanotide/mPEG-DSPE does not pose a carcinogenic risk to patients, with a safety margin of 17 for setmelanotide based on AUC and a dose margin of 16 for mPEG DSPE on a mg/m²/day basis, at the clinical dose of 3 mg/day. Due to the lack of pro-carcinogenic concern from the available non-clinical and clinical data on setmelanotide, a 2-year carcinogenicity study in rats has not been performed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.