IMITREX Film-coated tablet Ref.[10572] Active ingredients: Sumatriptan
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Sumatriptan binds with high affinity to human cloned 5‑HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine headache through agonist effects at the 5‑HT1B/1D receptors on intracranial blood vessels and sensory nerves of the trigeminal system, which result in cranial vessel constriction and inhibition of pro‑inflammatory neuropeptide release.
12.2. Pharmacodynamics
Blood Pressure
Significant elevation in blood pressure, including hypertensive crisis, has been reported in patients with and without a history of hypertension [see Warnings and Precautions (5.8)].
Peripheral (Small) Arteries
In healthy volunteers (N=18), a trial evaluating the effects of sumatriptan on peripheral (small vessel) arterial reactivity failed to detect a clinically significant increase in peripheral resistance.
Heart Rate
Transient increases in blood pressure observed in some patients in clinical trials carried out during sumatriptan’s development as a treatment for migraine were not accompanied by any clinically significant changes in heart rate.
12.3. Pharmacokinetics
Absorption
The mean maximum concentration following oral dosing with 25 mg is 18 ng/mL (range: 7 to 47 ng/mL) and 51 ng/mL (range: 28 to 100 ng/mL) following oral dosing with 100 mg of sumatriptan. This compares with a Cmax of 5 and 16 ng/mL following dosing with a 5- and 20‑mg intranasal dose, respectively. The mean Cmax following a 6‑mg subcutaneous injection is 71 ng/mL (range: 49 to 110 ng/mL). The bioavailability is approximately 15%, primarily due to presystemic metabolism and partly due to incomplete absorption. The Cmax is similar during a migraine attack and during a migraine‑free period, but the Tmax is slightly later during the attack, approximately 2.5 hours compared with 2.0 hours. When given as a single dose, sumatriptan displays dose proportionality in its extent of absorption (area under the curve [AUC]) over the dose range of 25 to 200 mg, but the Cmax after 100 mg is approximately 25% less than expected (based on the 25‑mg dose).
Effect of Food
A food effect trial involving administration of IMITREX tablets 100 mg to healthy volunteers under fasting conditions and with a high‑fat meal indicated that the Cmax and AUC were increased by 15% and 12%, respectively, when administered in the fed state.
Distribution
Protein binding, determined by equilibrium dialysis over the concentration range of 10 to 1,000 ng/mL is low, approximately 14% to 21%. The effect of sumatriptan on the protein binding of other drugs has not been evaluated. The apparent volume of distribution is 2.7 L/kg.
Metabolism
In vitro studies with human microsomes suggest that sumatriptan is metabolized by MAO, predominantly the A isoenzyme. Most of a radiolabeled dose of sumatriptan excreted in the urine is the major metabolite indole acetic acid (IAA) or the IAA glucuronide, both of which are inactive.
Elimination
The elimination half-life of sumatriptan is approximately 2.5 hours. Radiolabeled 14C-sumatriptan administered orally is largely renally excreted (about 60%) with about 40% found in the feces. Most of the radiolabeled compound excreted in the urine is the major metabolite, IAA, which is inactive, or the IAA glucuronide. Only 3% of the dose can be recovered as unchanged sumatriptan.
Specific Populations
Age
The pharmacokinetics of sumatriptan in the elderly (mean age: 72 years, 2 males and 4 females) and in subjects with migraine (mean age: 38 years, 25 males and 155 females) were similar to that in healthy male subjects (mean age: 30 years).
Patients with Renal Impairment
The effect of renal impairment on the pharmacokinetics of sumatriptan has not been examined.
Patients with Hepatic Impairment
The liver plays an important role in the presystemic clearance of orally administered sumatriptan. Accordingly, the bioavailability of sumatriptan following oral administration may be markedly increased in patients with liver disease. In one small trial of patients with moderate liver impairment (n=8) matched for sex, age, and weight with healthy subjects (n=8), the hepatically-impaired patients had an approximately 70% increase in AUC and Cmax and a Tmax 40 minutes earlier compared with the healthy subjects.
The pharmacokinetics of sumatriptan in patients with severe hepatic impairment has not been studied. The use of IMITREX tablets in this population is contraindicated [see Contraindications (4), Use in Specific Populations (8.6)].
Male and Female Patients
In a trial comparing females to males, no pharmacokinetic differences were observed between genders for AUC, Cmax, Tmax, and half‑life.
Racial Groups
The systemic clearance and Cmax of subcutaneous sumatriptan were similar in black (n=34) and Caucasian (n=38) healthy male subjects. Oral sumatriptan has not been evaluated for race differences.
Drug Interaction Studies
Monoamine Oxidase-A Inhibitors
Treatment with MAO-A inhibitors generally leads to an increase of sumatriptan plasma levels [see Contraindications (4), Drug Interactions (7.2)].
Due to gut and hepatic metabolic first-pass effects, the increase of systemic exposure after coadministration of an MAO-A inhibitor with oral sumatriptan is greater than after coadministration of the MAO inhibitors with subcutaneous sumatriptan.
In a trial of 14 healthy females, pretreatment with an MAO-A inhibitor decreased the clearance of subcutaneous sumatriptan, resulting in a 2-fold increase in the area under the sumatriptan plasma concentration-time curve (AUC), corresponding to a 40% increase in elimination half-life.
A small trial evaluating the effect of pretreatment with an MAO-A inhibitor on the bioavailability from a 25-mg oral sumatriptan tablet resulted in an approximately 7-fold increase in systemic exposure.
Alcohol
Alcohol consumed 30 minutes prior to sumatriptan ingestion had no effect on the pharmacokinetics of sumatriptan.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In carcinogenicity studies in mouse and rat, sumatriptan was administered orally for 78 and 104 weeks, respectively, at doses up to 160 mg/kg/day (the high dose in rat was reduced from 360 mg/kg/day during Week 21). There was no evidence in either species of an increase in tumors related to sumatriptan administration. Plasma exposures (AUC) at the highest doses tested were 20 and 8 times that in humans at the maximum recommended human dose (MRHD) of 200 mg/day.
Mutagenesis
Sumatriptan was negative in in vitro(bacterial reverse mutation [Ames], gene cell mutation in Chinese hamster V79/HGPRT, chromosomal aberration in human lymphocytes) and in vivo (rat micronucleus) assays.
Impairment of Fertility
When sumatriptan (5, 50, 500 mg/kg/day) was administered orally to male and female rats prior to and throughout the mating period, there was a treatment-related decrease in fertility secondary to a decrease in mating in animals treated with doses greater than 5 mg/kg/day (less than the MRHD on a mg/m² basis). It is not clear whether this finding was due to an effect on males or females or both.
When sumatriptan was administered by subcutaneous injection to male and female rats prior to and throughout the mating period, there was no evidence of impaired fertility at doses up to 60 mg/kg/day.
13.2. Animal Toxicology and/or Pharmacology
Corneal Opacities
Dogs receiving oral sumatriptan developed corneal opacities and defects in the corneal epithelium. Corneal opacities were seen at the lowest dose tested, 2 mg/kg/day, and were present after 1 month of treatment. Defects in the corneal epithelium were noted in a 60‑week study. Earlier examinations for these toxicities were not conducted and no‑effect doses were not established. Plasma exposure at the lowest dose tested was approximately 2 times that in humans at the MRHD.
14. Clinical Studies
The efficacy of IMITREX tablets in the acute treatment of migraine headaches was demonstrated in 3, randomized, double-blind, placebo-controlled trials. Patients enrolled in these 3 trials were predominately female (87%) and Caucasian (97%), with a mean age of 40 years (range: 18 to 65 years). Patients were instructed to treat a moderate to severe headache. Headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed up to 4 hours after dosing. Associated symptoms such as nausea, photophobia, and phonophobia were also assessed. Maintenance of response was assessed for up to 24 hours postdose. A second dose of IMITREX tablets or other medication was allowed 4 to 24 hours after the initial treatment for recurrent headache. Acetaminophen was offered to patients in Trials 2 and 3 beginning at 2 hours after initial treatment if the migraine pain had not improved or had worsened. Additional medications were allowed 4 to 24 hours after the initial treatment for recurrent headache or as rescue in all 3 trials. The frequency and time to use of these additional treatments were also determined. In all trials, doses of 25, 50, and 100 mg were compared with placebo in the treatment of migraine attacks. In 1 trial, doses of 25, 50, and 100 mg were also compared with each other.
In all 3 trials, the percentage of patients achieving headache response 2 and 4 hours after treatment was significantly greater among patients receiving IMITREX tablets at all doses compared with those who received placebo. In 1 of the 3 trials, there was a statistically significant greater percentage of patients with headache response at 2 and 4 hours in the 50-mg or 100-mg group when compared with the 25-mg dose groups. There were no statistically significant differences between the 50-mg and 100-mg dose groups in any trial. The results from the 3 controlled clinical trials are summarized in Table 2.
Table 2. Percentage of Patients with Headache Response (Mild or No Headache) 2 and 4 Hours following Treatment:
| IMITREX Tablets 25 mg | IMITREX Tablets 50 mg | IMITREX Tablets 100 mg | Placebo | |||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 2 h | 4 h | 2 h | 4 h | 2 h | 4 h | |
| Trial 1 | 52%a | 67%a | 61%a,b | 78%a,b | 62%a,b | 79%a,b | 27% | 38% |
| (n=298) | (n=296) | (n=296) | (n=94) | |||||
| Trial 2 | 52%a | 70%a | 50%a | 68%a | 56%a | 71%a | 26% | 38% |
| (n=66) | (n=62) | (n=66) | (n=65) | |||||
| Trial 3 | 52%a | 65%a | 54%a | 72%a | 57%a | 78%a | 17% | 19% |
| (n=48) | (n=46) | (n=46) | (n=47) | |||||
a P<0.05 in comparison with placebo.
b P<0.05 in comparison with 25 mg.
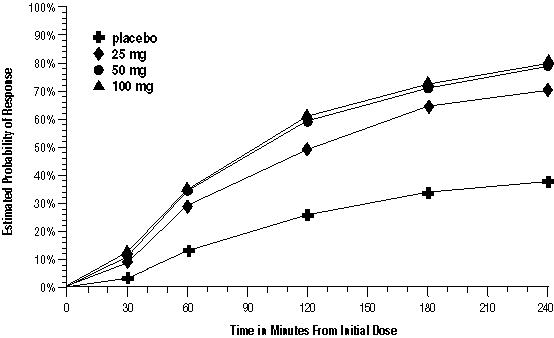
The estimated probability of achieving an initial headache response over the 4 hours following treatment in pooled Trials 1, 2, and 3 is depicted in Figure 1.
Figure 1. Estimated Probability of Achieving Initial Headache Response within 4 Hours of Treatment in Pooled Trials 1, 2, and 3a:
a The figure shows the probability over time of obtaining headache response (no or mild pain) following treatment with oral sumatriptan. The averages displayed are based on pooled data from the 3 clinical controlled trials providing evidence of efficacy. Kaplan‑Meier plot with patients not achieving response and/or taking rescue within 240 minutes censored to 240 minutes.
For patients with migraine-associated nausea, photophobia, and/or phonophobia at baseline, there was a lower incidence of these symptoms at 2 hours (Trial 1) and at 4 hours (Trials 1, 2, and 3) following administration of IMITREX tablets compared with placebo.
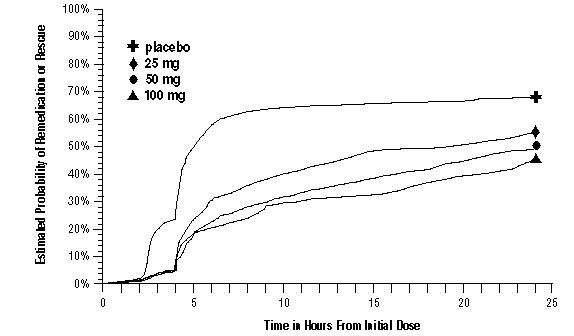
As early as 2 hours in Trials 2 and 3, or as early as 4 hours in Trial 1, through 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
Figure 2. The Estimated Probability of Patients Taking a Second Dose of IMITREX Tablets or Other Medication to Treat Migraine over the 24 Hours following the Initial Dose of Study Treatment in Pooled Trials 1, 2, and 3a:
a Kaplan‑Meier plot based on data obtained in the 3 clinical controlled trials providing evidence of efficacy with patients not using additional treatments censored to 24 hours. Plot also includes patients who had no response to the initial dose. No remedication was allowed within 2 hours postdose.
There is evidence that doses above 50 mg do not provide a greater effect than 50 mg. There was no evidence to suggest that treatment with IMITREX tablets was associated with an increase in the severity of recurrent headaches. The efficacy of IMITREX tablets was unaffected by presence of aura; duration of headache prior to treatment; gender, age, or weight of the subject; relationship to menses; or concomitant use of common migraine prophylactic drugs (e.g., beta-blockers, calcium channel blockers, tricyclic antidepressants). There were insufficient data to assess the impact of race on efficacy.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.