INDUCTOS Powder, solvent and matrix for implantation matrix Ref.[9360] Active ingredients: Dibotermin alfa
Source: European Medicines Agency (EU) Revision Year: 2018 Publisher: Medtronic BioPharma B.V., Earl Bakkenstraat 10, 6422 PJ Heerlen, The Netherlands, tel +31 (0) 45 566 8000, fax +31 (0) 45 566 8012
Therapeutic indications
InductOs is indicated for single-level lumbar interbody spine fusion as a substitute for autogenous bone graft in adults with degenerative disc disease who have had at least 6 months of non-operative treatment for this condition.
InductOs is indicated for the treatment of acute tibia fractures in adults, as an adjunct to standard care using open fracture reduction and intramedullary unreamed nail fixation.
See section 5.1.
Posology and method of administration
InductOs should be used by an appropriately qualified surgeon.
Posology
InductOs must be prepared exactly in accordance with the directions for preparation (see section 6.6).
The appropriate dose is determined by the volume of wetted matrix required for the intended indication.
If the surgical setting requires that only a portion of the product is needed, the wetted matrix should be cut to the desired size, and the unused portion must be discarded.
Dosing table for InductOs 4 mg pack:
| InductOs wetted matrices (4 mg pack) | Dimensions of wetted matrix | Volume of wetted matrix | Concentration of wetted matrix | Dibotermin alfa dose |
|---|---|---|---|---|
| 1 matrix | 2.5 cm x 5 cm | 1.3 cm³ | 1.5 mg/cm³ | 2 mg |
| 2 matrices | 2 x (2.5 cm x 5 cm) | 2.7 cm³ | 1.5 mg/cm³ | 4 mg |
Dosing table for InductOs 12 mg pack:
| Portion of InductOs wetted matrix (12 mg pack) | Dimensions of wetted matrix | Volume of wetted matrix | Concentration of wetted matrix | Dibotermin alfa dose |
|---|---|---|---|---|
| 1/6 of the matrix | 2.5 cm x 5 cm | 1.3 cm³ | 1.5 mg/cm³ | 2 mg |
| 1/3 of the matrix | 2.5 cm x 10 cm | 2.7 cm³ | 1.5 mg/cm³ | 4 mg |
| 2/3 of the matrix | 5 cm x 10 cm | 5.3 cm³ | 1.5 mg/cm³ | 8 mg |
| Entire matrix | 7.5 cm x 10 cm | 8 cm³ | 1.5 mg/cm³ | 12 mg |
Lumbar interbody fusion surgery
The required volume of InductOs is determined by the intervertebral disc space and the size, shape, and internal volume of the lumbar interbody fusion device(s) being used. Care must be taken not to compress the product or overfill the volume intended for new bone formation (see section 4.4).
Typically, 4 mg (2.7 cm 3 of wetted matrix) of InductOs is used in the intervertebral disc space. The maximum dosage is limited to 8 mg (5.3 cm 3 of wetted matrix) of InductOs in the intervertebral disc space. InductOs must be placed within the lumbar interbody fusion device(s) or in the anterior portion of the intervertebral disc space.
Acute tibia fracture surgery
The volume of InductOs to be implanted is determined by the fracture anatomy and the ability to close the wound without overly packing or compressing the product. Generally, each fracture site is treated with the contents of one 12 mg pack. The maximum dosage is limited to 24 mg (2 entire 12 mg pack matrices).
Paediatric population
The safety and efficacy of InductOs in children below 18 years of age have not been established. No data are available.
Method of administration
The medicinal product is administered by implantation.
For instructions on reconstitution of the medicinal product before administration, see section 6.6. Failure to follow the method of administration of InductOs may compromise its safety and efficacy.
Forceps should be used to handle InductOs. During handling and implantation, minimize fluid loss from the matrix. Do not squeeze.
Lumbar interbody fusion surgery
InductOs must not be used alone for this indication, but should be used with an approved (CE-marked) lumbar interbody fusion device(s). Compatibility has been demonstrated with titanium, polyetheretherketone (PEEK), and allograft bone.
Care and caution must be used to prevent overfilling the lumbar interbody fusion device and/or the anterior portion of the intervertebral disc space (see section 4.4).
Pre-Implantation
4 mg pack: The matrix is pre-cut in 2 pieces each of 2.5 × 5 cm.
12 mg pack: The matrix is in 1 piece of 7.5 cm x 10 cm. The wetted matrix should be cut into 6 equal pieces (approximately 2.5 × 5 cm) as an aid for dose selection. The selected pieces can be further cut as required.
The hollow geometry of the lumbar interbody fusion device must be carefully and loosely filled with the volume of InductOs corresponding to the internal volume of the device.
Implantation
As per standard practice, disc material and the cartilaginous portions of the vertebral endplates should be removed, preserving the cortical portions of the endplates, and haemostasis should be achieved (see section 4.5).
For instructions to implant the lumbar interbody fusion device, please refer to the manufacturer’s instructions for use.
InductOs must not be implanted posterior to the lumbar interbody fusion device where direct access to the spinal canal and/or nerve root(s) is possible. If leakage into the spinal canal and the nerve root is possible, a physical barrier between the matrix and any neurological tissue must be re-created by using, for example, local bone or allograft (see section 4.5).
Post-Implantation
Once InductOs and the lumbar interbody fusion device(s) are implanted, the inside of the intervertebral disc space must not be irrigated. Outside the intervertebral disc space, the surgical field should be irrigated as needed, and any fluid loss from the wetted matrix should be washed away.
If a surgical drain is required, the drain should be placed remotely from the implantation site or, preferably, one layer superficial to the implantation site.
Acute tibia fracture surgery
Pre-Implantation
Definitive fracture reduction, fixation, and haemostasis should be achieved prior to InductOs implantation.
InductOs should be folded or cut as needed prior to implantation. Implantation InductOs is implanted after the completion of standard fracture and wound management (i.e. at the time of soft-tissue closure).
To the extent possible, the accessible surface area of the fracture (fracture lines and defects) should be covered with InductOs. InductOs should be placed bridging the fracture region and making good contact with the major proximal and distal fragments.
InductOs may be placed into a void (loosely packed), folded, rolled or wrapped, as the geometry of the fracture requires. InductOs does not provide mechanical stability and should not be used to fill a void in the presence of compressive forces.
Post-Implantation
Once InductOs is implanted, do not irrigate the wound.
If a surgical drain is required, the drain should be placed remotely from the implantation site or, preferably, one layer superficially to the implantation site.
To achieve maximum potential efficacy, it is important to attain complete soft-tissue coverage of InductOs following its implantation.
Overdose
In case of overdose (i.e. a patient receives a concentration or amount of dibotermin alfa greater than recommended), treatment should be supportive.
Use of InductOs in patients undergoing cervical spine surgery in amounts lower than or similar to those for lumbar interbody fusion has been associated with reports of localised oedema severe enough to result in airway compromise (see section 4.4).
Shelf life
Shelf life: 3 years.
Special precautions for storage
Do not store above 30°C. Do not freeze.
Store in the original package in order to protect from light.
Nature and contents of container
InductOs 4 mg pack contains:
- Powder in a vial (10 ml; Type I glass) with a stopper (bromobutyl rubber).
- Solvent in a vial (10 ml; Type I glass) with a stopper (bromobutyl rubber).
- Two matrices (2.5 cm x 5 cm) in a blister package (polyvinyl chloride – PVC).
- Two syringes (5 ml; polypropylene).
- Two needles (stainless steel).
InductOs 12 mg pack contains:
- Powder in a vial (20 ml; Type I glass) with a stopper (bromobutyl rubber).
- Solvent in a vial (10 ml; Type I glass) with a stopper (bromobutyl rubber).
- One matrix (7.5 cm x 10 cm) in a blister package (polyvinyl chloride – PVC).
- Two syringes (10 ml; polypropylene).
- Two needles (stainless steel).
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
InductOs is prepared immediately prior to use. Dibotermin alfa must only be used following reconstitution with the solvent and matrix provided in the InductOs pack.
Once prepared, InductOs contains dibotermin alfa at a concentration of 1.5 mg/ml. InductOs must not be used in concentrations higher than 1.5 mg/ml (see section 4.9).
Product preparation
To prevent overloading the matrix, it is important to reconstitute the dibotermin alfa and to wet the entire matrix as described below.
4 mg pack:
In the non-sterile field:
1. Using sterile technique, place one syringe, one needle and the matrix inner package in the sterile field.
2. Disinfect the stoppers of the dibotermin alfa and solvent vials.
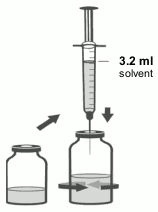
3. Using the remaining syringe and needle from the pack, reconstitute the dibotermin alfa vial with 3.2 ml of solvent. Slowly inject the solvent into the vial containing the lyophilised dibotermin alfa. Swirl the vial gently to aid reconstitution. Do not shake. Discard syringe and needle after use.
4. Disinfect the stopper of the reconstituted dibotermin alfa vial.
In the sterile field:
5. Peel open the interior package of the matrices and leave the matrices in their trays.
6. Using aseptic transfer technique and the syringe and needle from step 1, withdraw 2.8 ml of the reconstituted dibotermin alfa solution from the vial in the non-sterile field, holding up the inverted vial to facilitate withdrawal.
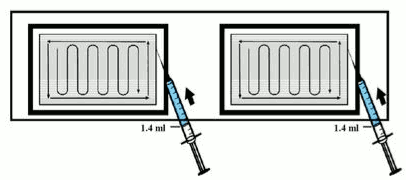
7. Leaving the matrix in its tray, UNIFORMLY distribute 1.4 ml of dibotermin alfa solution on each of the two 2.5 × 5 cm matrices, following the pattern in the figure below.
8. Wait a MINIMUM of 15 minutes before using the prepared InductOs product. The product must be used within 2 hours after preparation.
12 mg pack:
In the non-sterile field:
1. Using sterile technique, place one syringe, one needle and the matrix inner package in the sterile field.
2. Disinfect the stoppers of the dibotermin alfa and solvent vials.
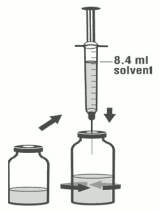
3. Using the remaining syringe and needle from the pack, reconstitute the dibotermin alfa vial with 8.4 ml of solvent. Slowly inject the solvent into the vial containing the lyophilised dibotermin alfa. Swirl the vial gently to aid reconstitution. Do not shake. Discard syringe and needle after use.
4. Disinfect the stopper of the reconstituted dibotermin alfa vial. In the sterile field.
5. Peel open the interior package of the matrix and leave the matrix in its tray.
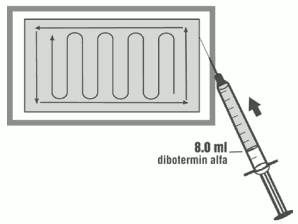
6. Using aseptic transfer technique and the syringe and needle from step 1, withdraw 8.0 ml of the reconstituted dibotermin alfa solution from the vial in the non-sterile field, holding up the inverted vial to facilitate withdrawal.
7. Leaving the matrix in its tray, UNIFORMLY distribute the dibotermin alfa solution on the matrix, following the pattern in the figure below.
8. Wait a MINIMUM of 15 minutes before using the prepared InductOs product. The product must be used within 2 hours after preparation.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.