ISOVUE 200/250/300/370 Solution for injection Ref.[10852] Active ingredients: Iopamidol
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
ISOVUE (lopamidol Injection) formulations are stable, aqueous, sterile, and nonpyrogenic solutions for intravascular administration.
Each mL of ISOVUE-200 (lopamidol Injection 41%) provides 408 mg iopamidol with 1 mg tromethamine and 0.26 mg edetate calcium disodium. The solution contains approximately 0.029 mg (0.001 mEq) sodium and 200 mg organically bound iodine per mL.
Each mL of ISOVUE-250 (lopamidol Injection 51%) provides 510 mg iopamidol with 1 mg tromethamine and 0. 33 mg edetate calcium disodium. The solution contains approximately 0.036 mg (0.002 mEq) sodium and 250 mg organically bound iodine per mL.
Each mL of ISOVUE-300 (lopamidol Injection 61%) provides 612 mg iopamidol with 1 mg tromethamine and 0.39 mg edetate calcium disodium. The solution contains approximately 0.043 mg (0.002 mEq) sodium and 300 mg organically bound iodine per mL.
Each mL of ISOVUE-370 (lopamidol Injection 76%) provides 755 mg iopamidol with 1 mg tromethamine and 0.48 mg edetate calcium disodium. The solution contains approximately 0.053 mg (0.002 mEq) sodium and 370 mg organically bound iodine per mL.
The pH of ISOVUE contrast media has been adjusted to 6.5-7.5 with hydrochloric acid and/or sodium hydroxide. Pertinent physicochemical data are noted below. ISOVUE (lopamidol Injection) is hypertonic as compared to plasma and cerebrospinal fluid (approximately 285 and 301 mOsm/kg water, respectively).
| Iopamidol | ||||
|---|---|---|---|---|
| Parameter | 41% | 51% | 61% | 76% |
| Concentration (mgl/mL) | 200 | 250 | 300 | 370 |
| Osmolality @37° C (mOsm/kg water) | 413 | 524 | 616 | 796 |
| Viscosity (cP) @ 37°C | 2.0 | 3.0 | 4.7 | 9.4 |
| @ 20°C | 3.3 | 5.1 | 8.8 | 20.9 |
| Specific Gravity @ 37°C | 1.227 | 1.281 | 1.339 | 1.405 |
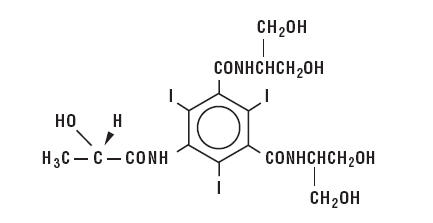
lopamidol is designated chemically as (S)-N,N'-bis[2-hydroxy-1-(hydroxymethyl)-ethyl]-2,4,6-triiodo-5-lactamidoisophthalamide.
Structural formula:
MW 777.09
C17H22I3N3O8
CAS-60166-93-0
Organically Bound Iodine: 49%
| How Supplied |
|---|
|
ISOVUE-200 (lopamidol Injection 41%): Ten 50 mL single dose vials (NDC 0270-1314-30) ISOVUE-250 (lopamidol Injection 51%): Ten 50 mL single dose vials (NDC 0270-1317-05) ISOVUE-300 (lopamidol Injection 61%): Ten 30 mL single dose vials (NDC 0270-1315-25) ISOVUE-370 (lopamidol Injection 76%): Ten 50 mL single dose vials (NDC 0270-1316-30) Also Available: Lopamidol Injection is also available as ISOVUE-M for intrathecal administration. Manufactured for Bracco Diagnostics Inc. - Monroe Township, NJ 08831 by BIPSO GmbH, 78224 Singen (Germany) |
Drugs
| Drug | Countries | |
|---|---|---|
| ISOVUE | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.